538329
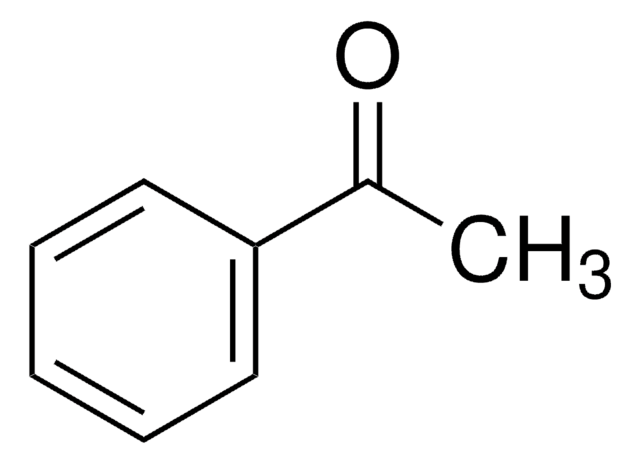
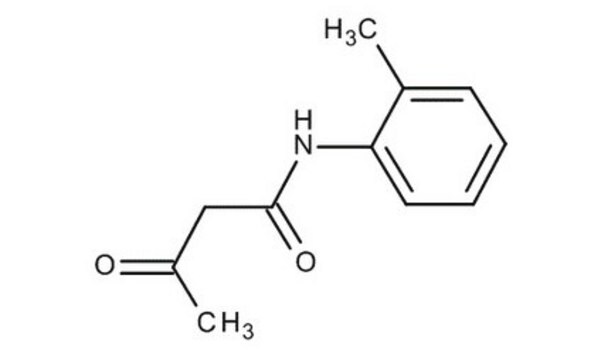
Acetoacetanilide
≥99.5%
Synonym(s):
1-(Phenylamino)-1,3-butanedione, 1-(Phenylcarbamoyl)-2-propanone, 3-Oxo-N-phenylbutanamide, 3-Oxo-N-phenylbutyramide, 4-(Phenylamino)-2,4-butanedione, Acetoacetamidobenzene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3COCH2CONHC6H5
CAS Number:
Molecular Weight:
177.20
Beilstein:
473419
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
6.1 (vs air)
Quality Level
Assay
≥99.5%
autoignition temp.
843 °F
mp
83-88 °C (lit.)
SMILES string
CC(=O)CC(=O)Nc1ccccc1
InChI
1S/C10H11NO2/c1-8(12)7-10(13)11-9-5-3-2-4-6-9/h2-6H,7H2,1H3,(H,11,13)
InChI key
DYRDKSSFIWVSNM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Acetoacetanilide (N-phenyl-3-oxobutanamide) undergoes condensation reaction with o-phenylenediamine to afford a Schiff base. Dielectric constant of acetoacetanilide crystals were found to increase on exposure to 120MeV Ag13+ ions due to increase in number of defects. Acetoacetanilide/Ce4+ system serves as an initiator during the polymerization of vinyl monomers.
Application
Acetoacetanilide may be used to synthesize:
- azo pigments
- acetoacetanilido-4-aminoantipyrine (Schiff base)
- 6-aryl-2-methyl-4-oxo-N,N′-diphenyl-2-cyclohexene-1,3-dicarboxamides
- photoluminescent lanthanide complexes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Crystal structures of azo pigments derived from acetoacetanilide
Whitaker, A.
Journal of the Society of Dyers and Colourists, 104.7-8 , 294-300 (1988)
Synthesis and photoluminescent properties of lanthanides acetoacetanilide complexes
Souza, E. R., et al.
Journal of Fluorescence, 23.5, 939-946 (2013)
Formation of 6-aryl-2-methyl-4-oxo-N, N?-diphenyl-2-cyclohexene-1, 3-dicarboxamides from acetoacetanilide and aromatic aldehydes catalyzed by a mixture of aryl amines and iodine
Gein VL, et al.
Russ. J. Gen. Chem., 86.1, 58-61 (2016)
Synthesis, spectral, redox and antimicrobial activities of Schiff base complexes derived from 1-phenyl-2, 3-dimethyl-4-aminopyrazol-5-one and acetoacetanilide
Raman N, et al.
Transition Metal Chemistry, 26.1-2, 131-135 (2001)
On the kinetics and initiation mechanism of acrylamide polymerization using the ceric ion/acetoacetanilide system as initiator
Dong JH, et al.
Macromolecular Chemistry and Physics, 195.3, 823-831 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service