530263
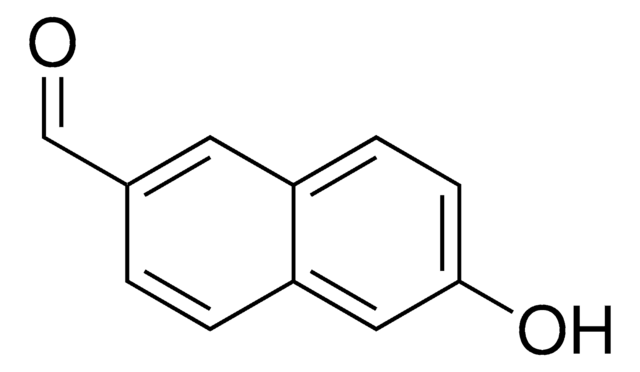
6-Cyano-2-naphthol
97%
Synonym(s):
2-Cyano-6-hydroxynaphthalene, 2-Cyano-6-naphthol, 2-Hydroxy-6-naphthonitrile, 6-Cyano-2-hydroxynaphthalene, 6-Hydroxy-2-naphthalenecarbonitrile, 6-Hydroxy-2-naphthonitrile
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NCC10H6OH
CAS Number:
Molecular Weight:
169.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
165.5-170.5 °C (lit.)
SMILES string
Oc1ccc2cc(ccc2c1)C#N
InChI
1S/C11H7NO/c12-7-8-1-2-10-6-11(13)4-3-9(10)5-8/h1-6,13H
InChI key
WKTNIBWKHNIPQR-UHFFFAOYSA-N
General description
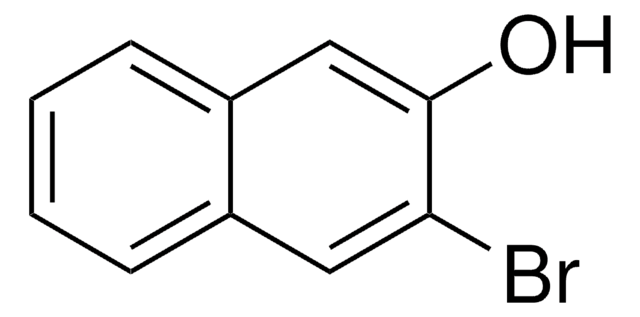
6-Cyano-2-naphthol (6CN2) is an aromatic alcohol that can be synthesized from 6-bromo-2-naphthol. It is a superphotoacid with the ground state pKa* value of 8.4 and excited state pKavalue of 0.2, respectively. 6CN2 protonates PANI-ES (polyaniline emeraldine salt) to form PANI-EB (emeraldine base), which shows enhanced conductivity. The proton-transfer kinetics and photophysical behavior of 6CN2 have been investigated.
Application
6-Cyano-2-naphthol (6-Hydroxy-2-naphthonitrile, 2-cyano-6-naphthol) may be used in the preparation of:
- 5-bromo-6-hydroxy-2-naphthonitrile
- 5,7-dibromo-6-hydroxy-2-naphthonitrile
- 5-chloro-6-hydroxy-2-naphthonitrile
- 6-(2-imidazolyl)-2-naphthol
- dodecaethylene glycol di-6-cyano-2-naphthyl ether
- 6-cyano-2-naphthyl trifluoremethanesufonate
- 2-(6-cyano-naphthyl)2,3,4-tri-O-acetyl-β-D-xylopyranoside
- 1,5-bis(7-amidino-2-naphthalenoxy)-3-oxapentane dihydrochloride
Reactant for:
- Palladium-catalyzed reduction
- Nickel-catalyzed cross-coupling reactions
- Palladium-catalyzed Heck reactions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T Nakayama et al.
Chemical & pharmaceutical bulletin, 41(1), 117-125 (1993-01-01)
By developing 6-amidino-2-naphthyl 4-guanidinobenzoate (I, FUT-175) as a basic structure, its various derivatives were synthesized and their inhibitory activities on trypsin, plasmin, kallikrein, thrombin, C1r and C1s as well as on complement-mediated hemolysis were examined. The protective effect of these
ssDNA templated assembly of oligonucleotides and bivalent naphthalene guests.
Janssen PGA, et al.
Soft Matter, 6(7), 1494-1502 (2010)
Maryam Rahimian et al.
Biochemistry, 48(7), 1573-1583 (2009-01-29)
Most A/T specific heterocyclic diamidine derivatives need at least four A/T base pairs for tight binding to the DNA minor groove. Addition of a GC base pair to A/T sequences typically causes a large decrease in binding constant. The ability
S Ono et al.
Chemical & pharmaceutical bulletin, 47(12), 1685-1693 (2000-04-05)
The synthesis and design using molecular modeling techniques for non-peptide, low molecular weight novel fibrinogen receptor (glycoprotein IIb/IIIa: Gp IIb/IIIa) antagonists, is reported. We used a highly potent serine protease inhibitor, Nafamostat, having an amidinonaphthyl unit as the starting compound.
Yong-Hong Liang et al.
Bioorganic & medicinal chemistry, 18(13), 4601-4605 (2010-06-24)
Nine newly 6-cyano-2-naphthyl substituted diarylpyrimidines (DAPY) were synthesized as non-nucleoside reverse transcriptase inhibitors on the basis of our previous work. The antiviral and cytotoxicity evaluation indicated that these compounds displayed strong activity against wild-type HIV-1 at nanomolar concentrations with selectivity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service