520330
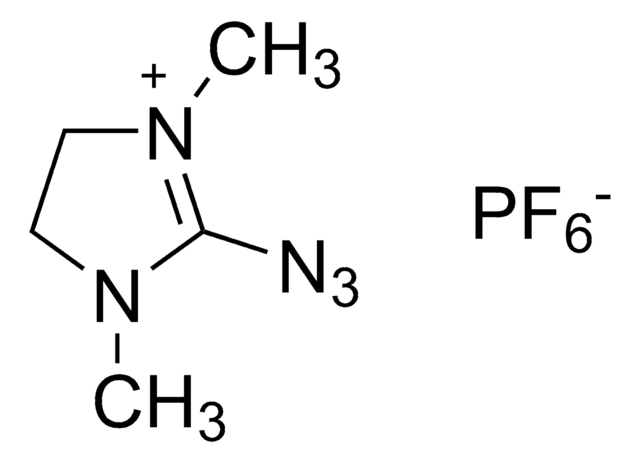
Fluoro-N,N,N′,N′-tetramethylformamidinium hexafluorophosphate
97%
Synonym(s):
TFFH
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
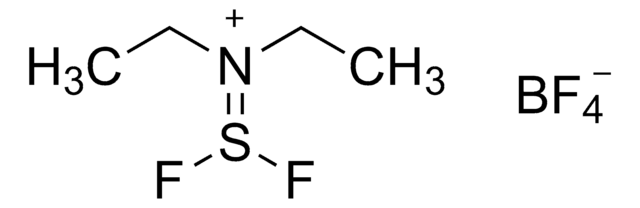
[FC[=N(CH3)2]N(CH3)2]PF6
CAS Number:
Molecular Weight:
264.12
MDL number:
UNSPSC Code:
12352107
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
reaction suitability
reaction type: Coupling Reactions
mp
104-109 °C (lit.)
application(s)
peptide synthesis
SMILES string
F[P-](F)(F)(F)(F)F.CN(C)\C(F)=[N+](/C)C
InChI
1S/C5H12FN2.F6P/c1-7(2)5(6)8(3)4;1-7(2,3,4,5)6/h1-4H3;/q+1;-1
InChI key
ZAVXOOLKAGPJPI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Reagent for synthesis of:
Cationic antimicrobial β-2,2-amino acid derivatives
Philanthotoxin analogues for inhibition of the ionotropic glutamate receptor
Small β-peptidomimetics for antimicrobials
Amine prodrugs of selective neuronal nitric oxide synthase inhibitors
Reagent for:
Selective cyclohexyl-derived imidazopyrazine insulin-like growth factor 1 receptor inhibitors
Hydrazone ligation to assemble multifunctional viral nanoparticles
Cationic antimicrobial β-2,2-amino acid derivatives
Philanthotoxin analogues for inhibition of the ionotropic glutamate receptor
Small β-peptidomimetics for antimicrobials
Amine prodrugs of selective neuronal nitric oxide synthase inhibitors
Reagent for:
Selective cyclohexyl-derived imidazopyrazine insulin-like growth factor 1 receptor inhibitors
Hydrazone ligation to assemble multifunctional viral nanoparticles
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anita K Kovács et al.
Frontiers in chemistry, 6, 120-120 (2018-05-05)
A general strategy for the synthesis of N-peptide-6-amino-D-luciferin conjugates has been developed. The applicability of the strategy was demonstrated with the preparation of a known substrate, N-Z-Asp-Glu-Val-Asp-6-amino-D-luciferin (N-Z-DEVD-aLuc). N-Z-DEVD-aLuc was obtained via a hybrid liquid/solid phase synthesis method, in which
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service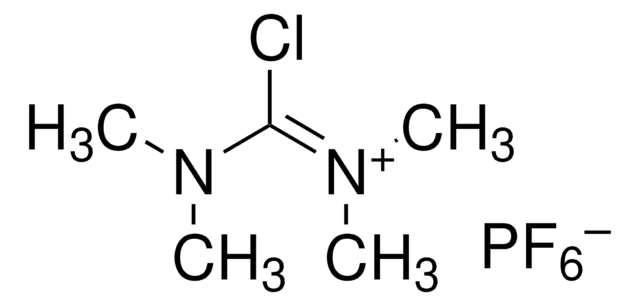







![COMU 1-[(1-(Cyano-2-ethoxy-2-oxoethylideneaminooxy) dimethylaminomorpholino)] uronium hexafluorophosphate Novabiochem®](/deepweb/assets/sigmaaldrich/product/images/237/337/13566c06-8931-4cc2-8621-c8742a392cd6/640/13566c06-8931-4cc2-8621-c8742a392cd6.jpg)