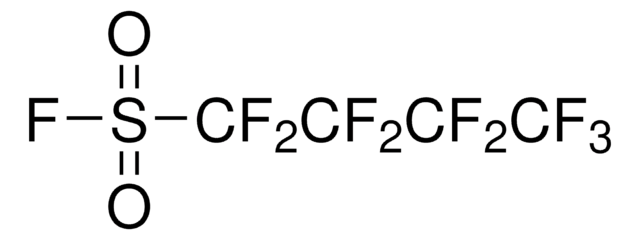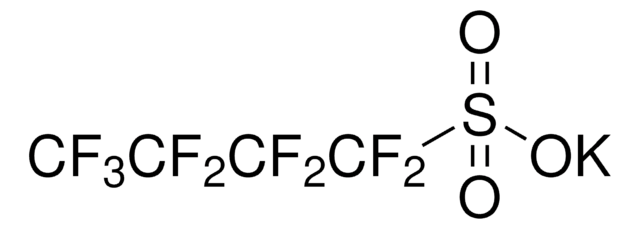51974
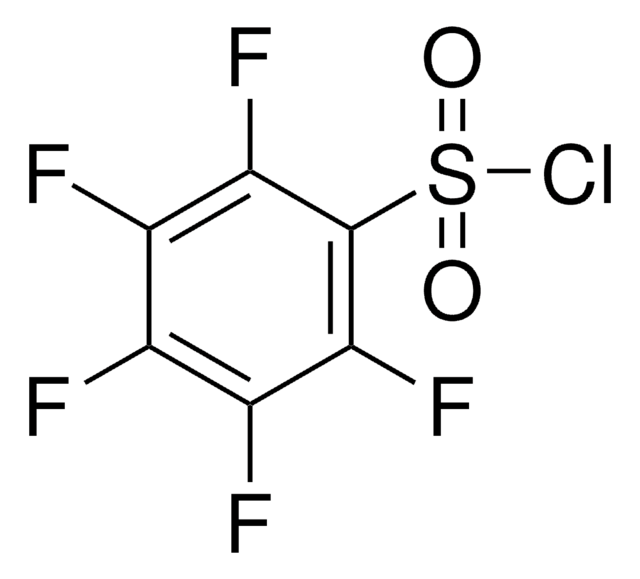
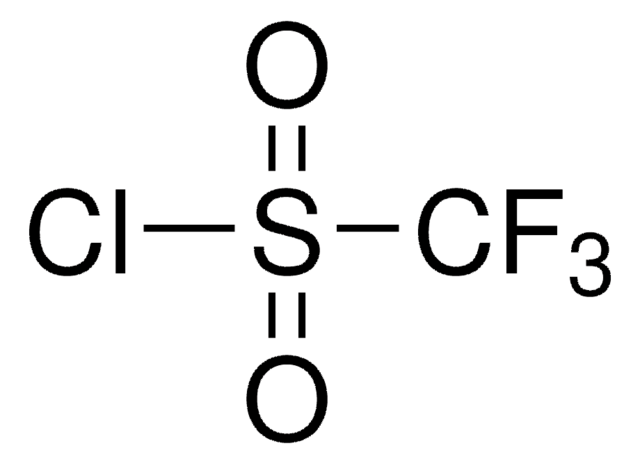
Nonafluoro-1-butanesulfonyl chloride
≥98.0% ((GC))
Synonym(s):
Perfluoro-1-butanesulfonyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4ClF9O2S
CAS Number:
Molecular Weight:
318.55
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% ((GC))
SMILES string
FC(F)(F)C(F)(F)C(F)(F)C(F)(F)S(Cl)(=O)=O
InChI
1S/C4ClF9O2S/c5-17(15,16)4(13,14)2(8,9)1(6,7)3(10,11)12
InChI key
IRFCLLARAUQTNK-UHFFFAOYSA-N
Other Notes
Reagent for fluoro-tagging of nucleophiles
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C.W. Lindsley et al.
Tetrahedron Letters, 43, 4225-4225 (2002)
Graphitic carbon nitride polymer as a recyclable photoredox catalyst for fluoroalkylation of arenes.
Moritz Baar et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(2), 526-530 (2014-11-22)
Heterogeneous catalysis for trifluoromethylations and perfluoroalkylations has been performed. Through the usage of cheap, metal-free and recyclable mesoporous graphitic carbon nitride (mpg-CN) it was possible to fluoroalkylate various arenes by the reductive activation of sulfonyl chlorides with visible light. Thus
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service