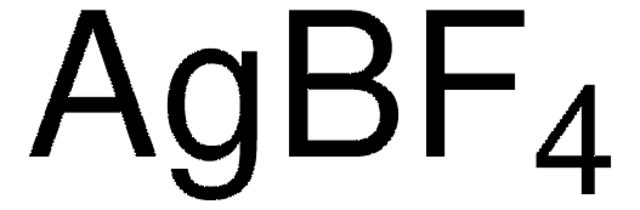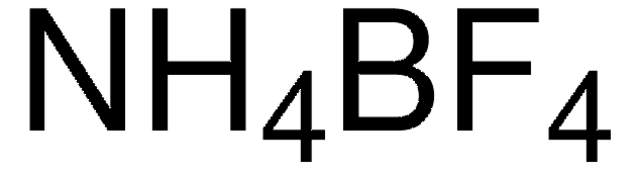483052
Silver tetrafluoroborate
≥99.99% trace metals basis
Synonym(s):
Silver borofluoride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
AgBF4
CAS Number:
Molecular Weight:
194.67
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
≥99.99% trace metals basis
form
powder
reaction suitability
reagent type: catalyst
core: silver
impurities
≤100.0 ppm Trace Metal Analysis
mp
70-73 °C (lit.)
SMILES string
[Ag+].F[B-](F)(F)F
InChI
1S/Ag.BF4/c;2-1(3,4)5/q+1;-1
InChI key
CCAVYRRHZLAMDJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zbigniew J Kamiński et al.
Journal of the American Chemical Society, 127(48), 16912-16920 (2005-12-01)
A new generation of triazine-based coupling reagents (TBCRs), designed according to the concept of "superactive esters", was obtained by treatment of 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium (DMTMM) chloride with lithium or silver tetrafluoroborate. The structure of 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium tetrafluoroborate was confirmed by X-ray diffraction. Activation
M Yoshida et al.
Chemical & pharmaceutical bulletin, 38(1), 273-275 (1990-01-01)
Silver tetrafluoroborate (AgBF4) in trifluoroacetic acid (TFA) has been found to cleave the S-trimethylacetamidomethyl (Tacm) group or the S-acetamidomethyl (Acm) group without affecting other functional groups in the peptide chain. Newly isolated porcine brain natriuretic peptide-32 (pBNP-32) was synthesized using
Matthew C Achilonu et al.
Organic letters, 10(17), 3865-3868 (2008-08-06)
A novel and efficient method for the oxidative condensation of tetra-O-methyl-3-oxocatechin 4 with tetra-O-methylcatechin is described. Treatment of a solution of 3 (2 equiv) and 4 (1 equiv) with silver tetrafluoroborate readily affords the phenolic per-O-methyl ethers of 3-oxocatechin(4-8)-catechin 18
M Yoshida et al.
Chemical & pharmaceutical bulletin, 38(6), 1551-1557 (1990-06-01)
Silver tetrafluoroborate (AgBF4) in trifluoroacetic acid (TFA) has been found to cleave the S-trimethyl-acetamidomethyl (Tacm) group or the S-acetamidomethyl (Acm) group without affecting other functional groups in a peptide chain. A newly isolated porcine brain natriuretic peptide-32 (pBNP-32) was synthesized
Min Li et al.
Talanta, 78(4-5), 1364-1370 (2009-04-14)
The extraction/enrichment of omega-3 polyunsaturated fatty acid methyl esters (PUFAMEs) by hydrophobic ionic liquids (ILs) containing silver salts as the extraction phase has been extended to include equilibrium studies. The extraction time, organic solvents, IL structures, and AgBF4 concentrations all
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service