479403
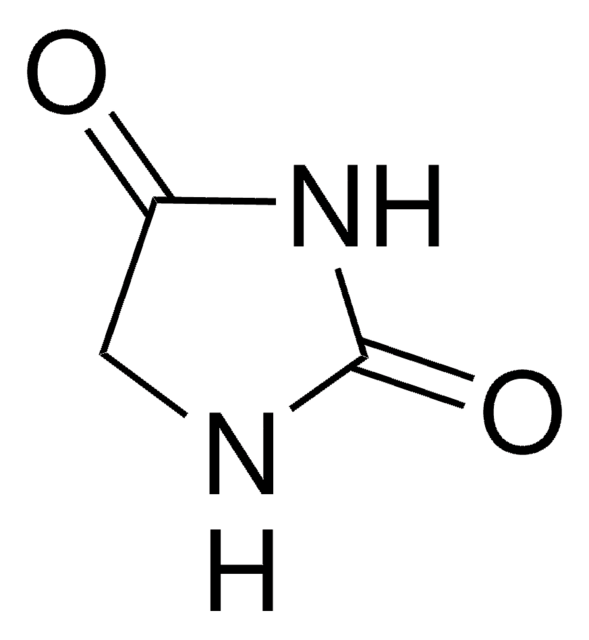
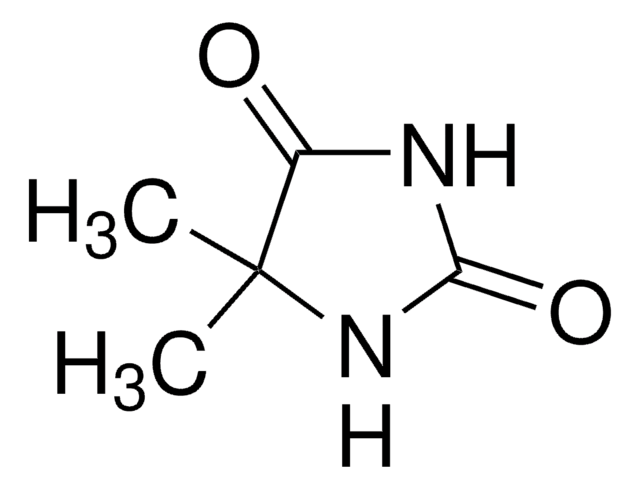
1,5,5-Trimethylhydantoin
98%
Synonym(s):
1,5,5-Trimethyl-2,4-imidazolidinedione, 3,4,4-Trimethyl-2,5-dioxoimidazolidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H10N2O2
CAS Number:
Molecular Weight:
142.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
161-164 °C (lit.)
SMILES string
CN1C(=O)NC(=O)C1(C)C
InChI
1S/C6H10N2O2/c1-6(2)4(9)7-5(10)8(6)3/h1-3H3,(H,7,9,10)
InChI key
ZNYIPTYJBRGSSL-UHFFFAOYSA-N
General description
1,5,5-Trimethylhydantoin (TMH) is a 1,5,5-trisubstituted hydantoin. Its mass spectrum has been recorded and analyzed. The density of TMH is 1.1318g/ml at 25°C.
Application
1,5,5-Trimethylhydantoin (1,5,5-Trimethyl-imidazolidine-2,4-dione) may be used to synthesize 3-bromomethyl-1,5,5-trimethylimidazolid-ine-2,4-dione.
Reactant for:
Z-selective hydroamidation of terminal alkynes with secondary amides and imides
Selective inhibitors of hepatitis C virus NS3 serine protease
Stereoselective addition of imides to alkynes
Reactant for synthesis of:
Selective angiotensin II AT2 receptor agonists with reduced CYP 450 inhibition
N-chlorohydantoins
P2X7 receptor antagonists
Z-selective hydroamidation of terminal alkynes with secondary amides and imides
Selective inhibitors of hepatitis C virus NS3 serine protease
Stereoselective addition of imides to alkynes
Reactant for synthesis of:
Selective angiotensin II AT2 receptor agonists with reduced CYP 450 inhibition
N-chlorohydantoins
P2X7 receptor antagonists
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mass spectrometric analysis and theoretical calculations of the occurrence of tautomeric structures of hydantoins.
Allegretti PE, et al.
Afinidad, 57(485), 41-49 (2000)
Use of the cascade α-oxo-amidoalkylation/transposition/Π-cationic cyclization of N-acyliminium ions in the synthesis of novel fused heterocyclic N,O-acetals.
Pesquet A, et al.
ARKIVOC (Gainesville, FL, United States), 8, 27-40 (2010)
Yaws CL.
Thermophysical Properties of Chemicals and Hydrocarbons, 280-280 (2008)
Zi-Ao Huang et al.
Electrophoresis, 41(3-4), 183-193 (2019-12-19)
In this paper, the development of a simple dilute-and-shoot method for quantifying urinary creatinine by CE-ESI-MS was described. The creatinine analysis time was about 7 min/sample by conventional single injection (SI) method and can be significantly reduced to less than 2 min/sample
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service