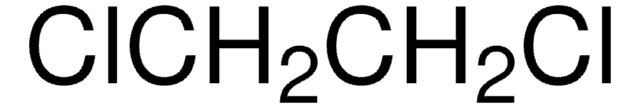47649
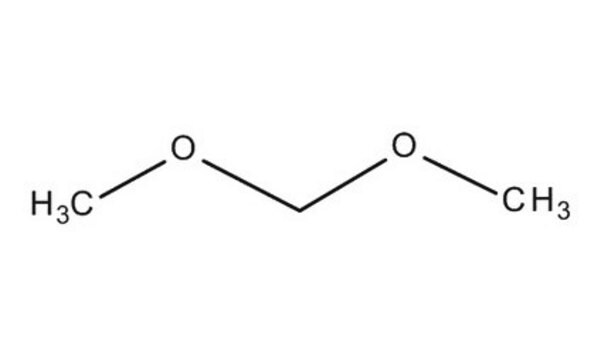
Formaldehyde dimethyl acetal
for Grignard reactions, ≥99.0% (GC)
Synonym(s):
Dimethoxymethane, Methylal
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH2(OCH3)2
CAS Number:
Molecular Weight:
76.09
Beilstein:
1697025
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0% (GC)
reaction suitability
reaction type: Grignard Reaction
impurities
≤0.2% water
density
0.860 g/mL at 20 °C (lit.)
SMILES string
COCOC
InChI
1S/C3H8O2/c1-4-3-5-2/h3H2,1-2H3
InChI key
NKDDWNXOKDWJAK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Formaldehyde dimethyl acetal (FDA, FDMA, FADMA, dimethoxymethane, DMM, methylal) is a biodegradable dimethyl acetal. It has been synthesized by condensing formaldehyde with methanol in the presence of acid catalyst. It is ampiphilic in nature with low viscosity, surface tension and boiling point. It is a flammable, highly volatile solvent with excellent dissolving power. It is reported to form trimethylorthoformate by anodic methoxylation in basic methanol.
Application
Formaldehyde dimethyl acetal may be used in the following studies:
- Synthesis of methoxymethyl (MOM) ethers.
- As an external cross-linker to form microporous polymers.
- Dehydration of biological samples for scanning electron microscopy (SEM).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
-0.4 °F - closed cup
Flash Point(C)
-18 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Electrosynthesis of trimethylorthoformate on BDD electrodes.
Fardel R, et al.
J. Appl. Electrochem., 36(2), 249-253 (2006)
Calceolariaceae: floral development and systematic implications.
Mayr EM and Weber A.
American Journal of Botany, 93(3), 327-343 (2006)
Purification and dehydration of methylal by pervaporation.
Carretier E, et al.
Journal of Membrane Science, 217(1), 159-171 (2003)
A new strategy to microporous polymers: knitting rigid aromatic building blocks by external cross-linker.
Li B, et al.
Macromolecules, 44(8), 2410-2414 (2011)
Scandium trifluoromethanesulfonate as a recyclable catalyst for efficient methoxymethylation of alcohols.
Karimi B and Ma'mani L.
Tetrahedron Letters, 44(32), 6051-6053 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service