All Photos(1)
About This Item
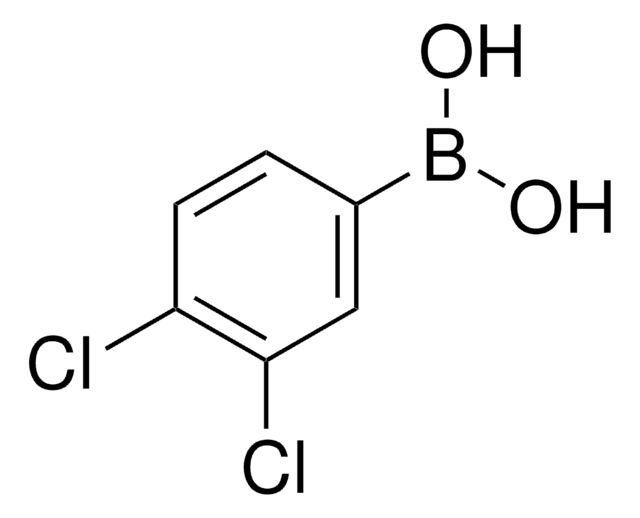
Linear Formula:
F2C6H3B(OH)2
CAS Number:
Molecular Weight:
157.91
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
147-149 °C (lit.)
SMILES string
OB(O)c1c(F)cccc1F
InChI
1S/C6H5BF2O2/c8-4-2-1-3-5(9)6(4)7(10)11/h1-3,10-11H
InChI key
DBZAICSEFBVFHL-UHFFFAOYSA-N
Related Categories
Application
2,6-Difluorophenylboronic acid can be used:
- As a substrate in the model reaction of Suzuki–Miyaura coupling with 4-chloro-3-methylanisole.
- To prepare 4-bromo-2,3′,5′,6-tetrafluorobiphenyl, a key intermediate for the synthesis of 2,6-difluorinated oligophenyls applicable in organic semiconductors.
- To prepare ethyl 4-(2,6-difluorophenyl)nicotinate, a key intermediate for the synthesis of 4-phenyl pyridine based potent TGR5 agonists.
Other Notes
Contains varying amounts of the anhydride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Enhancing charge mobilities in selectively fluorinated oligophenyl organic semiconductors: a design approach based on experimental and computational perspectives
Maiti B, et al.
Journal of Material Chemistry C, 7(13), 3881-3888 (2019)
Design and preparation of new palladium precatalysts for C-C and C-N cross-coupling reactions
Bruno NC, et al.
Chemical Science, 4(3), 916-920 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service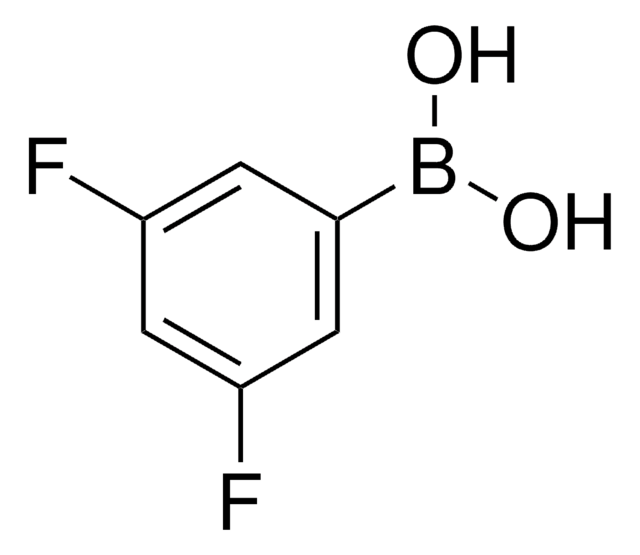

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)