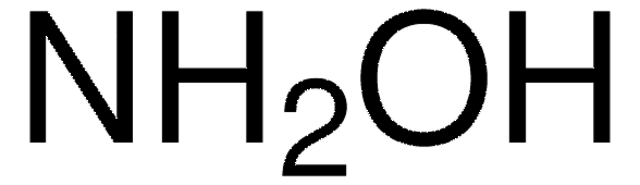467804
Hydroxylamine solution
50 wt. % in H2O, 99.999%
Synonym(s):
HDA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2OH
CAS Number:
Molecular Weight:
33.03
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99.999%
concentration
50 wt. % in H2O
bp
107 °C
density
1.078 g/mL at 25 °C
SMILES string
NO
InChI
1S/H3NO/c1-2/h2H,1H2
InChI key
AVXURJPOCDRRFD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Hydroxylamine solution is widely used as a reducing agent and antioxidant in organic synthesis, polymer industry and water treatment.
Application
Hydroxylamine solution (NH2OH) can be used as a reactant for the preparation of:
- Primary amides from aldehydes catalyzed by an arene–ruthenium(II) complex.
- Hydroxyaminoguanidines and carboxamide derivatives of ofloxacin for biological studies.
- Fe3O4/Au (GoldMag) nanoparticles for antibody immobilization.
Reactant for preparation of:
- Prodrug for cardiovascular agent Nω-hydroxy-L-arginine (NOHA, nitric oxide precursor)
- Hydroxyaminoguanidines as anti-cancer agents
- Nonsteroidal 2,3-dihydroquinoline glucocorticoid receptor agonists with reduced phosphoenolpyruvate caboxykinase (PEPCK) transactivation
- Carboxamide derivatives of ofloxacin with improved antimicrobial properties
- Analogues of coumarin based TNF-α converting enzyme (TACE) inhibitors
- HIV integrase inhibitors
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Carc. 2 - Desen. Expl. 4 - Eye Dam. 1 - Met. Corr. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 - STOT SE 3
Target Organs
Blood, Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ruthenium-catalyzed one-pot synthesis of primary amides from aldehydes in water
Garcia-Alvarez R, et al.
Royal Society of Chemistry Advances, 3(17), 5889-5894 (2013)
Synthesis, characterization and biological activity of a series of carboxamide derivatives of ofloxacin
Arayne MS, et al.
Archives of Pharmacal Research, 33(12), 1901-1909 (2010)
N-Hydroxy-N′-aminoguanidines as anti-cancer lead molecule: QSAR, synthesis and biological evaluation
Basu A, et al.
Bioorganic & Medicinal Chemistry Letters, 21(11), 3324-3328 (2011)
The synthesis of GoldMag nano-particles and their application for antibody immobilization
Cui Y, et al.
Biomedical Microdevices, 7(2), 153-156 (2005)
A E Cribb et al.
Clinical pharmacology and therapeutics, 51(5), 522-526 (1992-05-01)
The oxidation of sulfamethoxazole to its hydroxylamine metabolite was investigated in vitro with human liver microsomes and in vivo by detection in the urine. Sulfamethoxazole was oxidized to the hydroxylamine in an NADPH-dependent process by liver microsomes prepared from two
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service