441279
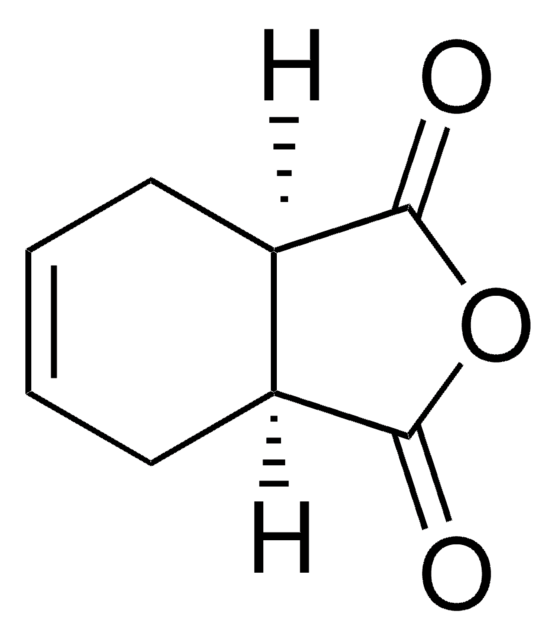
(1R)-(−)-2-Azabicyclo[2.2.1]hept-5-en-3-one
≥98%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H7NO
CAS Number:
Molecular Weight:
109.13
Beilstein:
4230721
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
solid
optical activity
[α]20/D −565°, c = 1 in chloroform
optical purity
ee: 99% (HPLC)
mp
94-97 °C (lit.)
SMILES string
O=C1N[C@@H]2C[C@H]1C=C2
InChI
1S/C6H7NO/c8-6-4-1-2-5(3-4)7-6/h1-2,4-5H,3H2,(H,7,8)/t4-,5+/m1/s1
InChI key
DDUFYKNOXPZZIW-UHNVWZDZSA-N
General description
(1R)-(-)-2-Azabicyclo[2.2.1]hept-5-en-3-one is a bicyclic γ-lactam.
Application
(1R)-(-)-2-Azabicyclo[2.2.1]hept-5-en-3-one can be used as a precursor to prepare:
- Amino-peramivir, a potent neuraminidase inhibitor.
- Five membered analogs of 4-amino-5-halopentanoic acids as potential GABA aminotransferase (GABA-AT) inactivators.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The de-guanidinylated derivative of peramivir remains a potent inhibitor of influenza neuraminidase
Bromba CM, et al.
Bioorganic & medicinal chemistry letters, 21(23), 7137-7141 (2011)
2-Azabicyclo [2.2. 1] hept-5-en-3-one: chemical profile of a versatile synthetic building block and its impact on the development of therapeutics.
Singh R and Vince R.
Chemical Reviews, 112(8), 4642-4686 (2012)
A new class of conformationally rigid analogues of 4-amino-5-halopentanoic acids, potent inactivators of ?-aminobutyric acid aminotransferase.
Qiu J and Silverman RB.
Journal of Medicinal Chemistry, 43(4), 706-720 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![2-Azabicyclo[2.2.1]hept-5-en-3-one 98%](/deepweb/assets/sigmaaldrich/product/structures/155/017/1874f631-2345-407a-83c6-4ef0fa3f35a3/640/1874f631-2345-407a-83c6-4ef0fa3f35a3.png)
![(1S)-(+)-2-Azabicyclo[2.2.1]hept-5-en-3-one ≥98%](/deepweb/assets/sigmaaldrich/product/structures/230/141/310ba46c-6b75-4ed3-9134-10ae806b9cc0/640/310ba46c-6b75-4ed3-9134-10ae806b9cc0.png)

![3-Amino-3-azabicyclo[3.3.0]octane hydrochloride 97%](/deepweb/assets/sigmaaldrich/product/structures/949/314/492dcd65-9f2b-4941-a3a1-07466ecdde74/640/492dcd65-9f2b-4941-a3a1-07466ecdde74.png)