441252
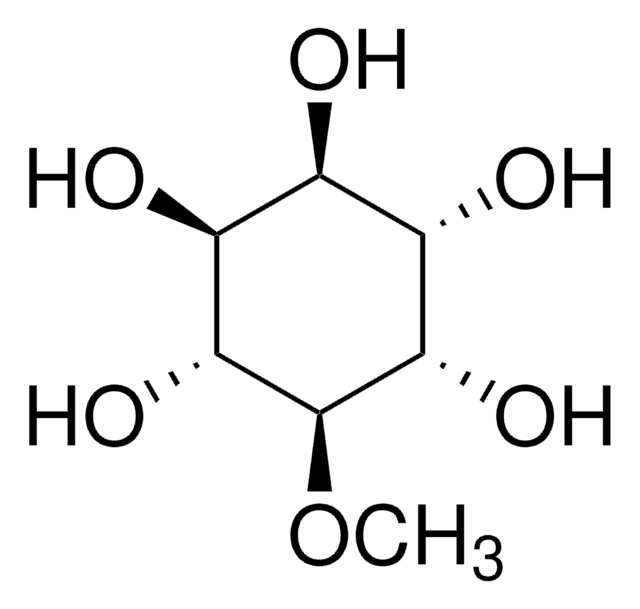
D-Pinitol
95%
Synonym(s):
3-O-Methyl-D-chiro-inositol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H14O6
CAS Number:
Molecular Weight:
194.18
MDL number:
UNSPSC Code:
12352112
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
powder
optical activity
[α]20/D 60.0 to 70.0°, c = 1% in H2O
mp
179-185 °C (lit.)
SMILES string
CO[C@@H]1[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)[C@H]1O
InChI
1S/C7H14O6/c1-13-7-5(11)3(9)2(8)4(10)6(7)12/h2-12H,1H3/t2-,3-,4-,5-,6+,7+/m0/s1
InChI key
DSCFFEYYQKSRSV-KLJZZCKASA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
D-pinitol, commonly found conifers, is an isomer of L-quebrachitol.
Application
D-pinitol may be used as a starting material to prepare its azole nucleoside analogs. It may also be used in the preparation of 1D-1,5-dideoxy-1,5-difluoro-neo-inositol and 1D-1-deoxy-1-fluoro-myo-inositol.
Precursor to biologically active fluorinated isosteres of inositol, which show cell growth inhibitory properties. Has shown antidiabetic properties in mice. Believed to be a salt stress regulator in a wide range of plants.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Narayanan, C.R. et al.
Current Science, 56, 139-139 (1987)
Efficient synthetic routes to fluorinated isosteres of inositol and their effects on cellular growth.
Kozikowski AP, et al.
Journal of the American Chemical Society, 112(11), 4528-4531 (1990)
Joanna Magielse et al.
Journal of ethnopharmacology, 146(1), 250-256 (2013-01-08)
The isolation of D-pinitol (or 3-O-methyl-D-chiro-inositol) from an aqueous decoction of Desmodium adscendens (Fabaceae) leaves and twigs is reported. The protective and curative effect of this decoction, in which d-pinitol was quantified, and of pure D-pinitol, against chemically-induced liver damage
Selvaraj Sivakumar et al.
Chemico-biological interactions, 188(1), 237-245 (2010-07-21)
Oxidative stress plays a crucial role in the progression and development of diabetes and its complications due to chronic hyperglycemia. The present study was aimed to investigate the kidney tissue protective nature of d-pinitol, a cyclitol present in soybean, by
Binika Hada et al.
The journals of gerontology. Series A, Biological sciences and medical sciences, 68(3), 226-234 (2012-07-31)
D-chiro-inositol, a member of the inositol family, and pinitol, a 3-methoxy analogue of D-chiro-inositol, have been proposed to have antidiabetic, antiinflammatory, anticancer and stamina enhancing effects. We found that supplementing the diet of Drosophila with D-chiro-inositol and pinitol extended adult
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service