430722
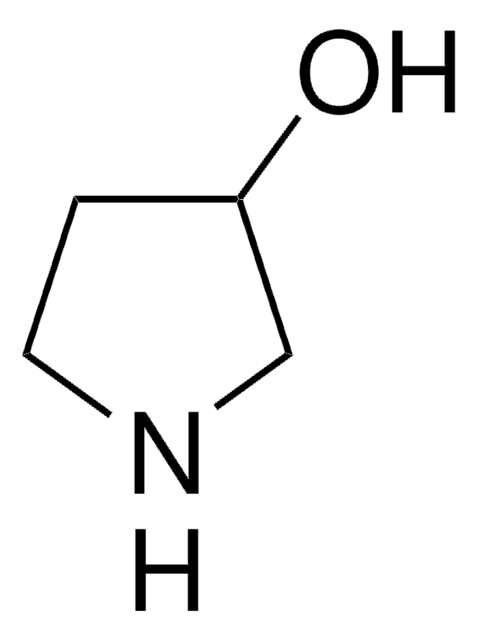
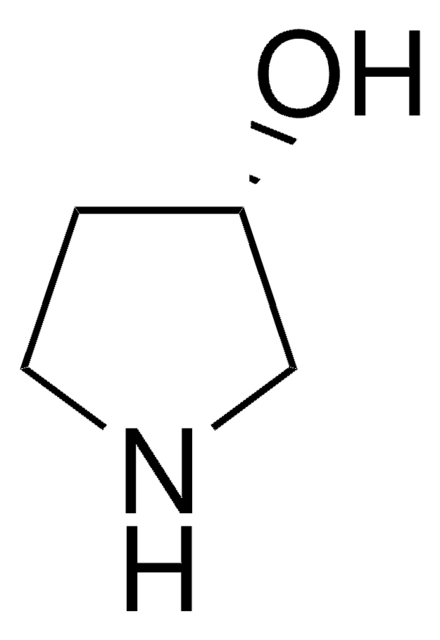
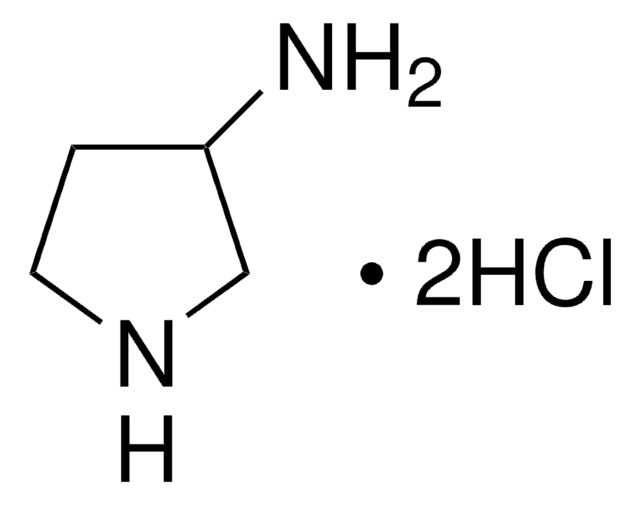
(R)-3-Pyrrolidinol hydrochloride
98%
Synonym(s):
(R)-3-Hydroxypyrrolidine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H9NO · HCl
CAS Number:
Molecular Weight:
123.58
Beilstein:
4450078
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
optical activity
[α]20/D −7.6°, c = 3.5 in methanol
mp
104-107 °C (lit.)
SMILES string
Cl.O[C@@H]1CCNC1
InChI
1S/C4H9NO.ClH/c6-4-1-2-5-3-4;/h4-6H,1-3H2;1H/t4-;/m1./s1
InChI key
QPMSJEFZULFYTB-PGMHMLKASA-N
Looking for similar products? Visit Product Comparison Guide
Application
(R)-3-Pyrrolidinol hydrochloride can be used as a building block to synthesize:
- Biaryl carboxamide functional groups containing bis-aminopyrrolidine ureas as potential antagonists of the melanin-concentrating hormone receptor-1.
- Pyrrolidinol based ionic liquids.
- Optically active 2-tritylpyrrolidine based organocatalysts.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Commercially available salts as building blocks for new ionic liquids
Davis JH and Fox PA
ACS Symposium Series, 15(14), 3439-3445 (2003)
A Recyclable Chiral 2-(Triphenylmethyl) pyrrolidine Organocatalyst Anchored to [60] Fullerene
Rosso C, et al.
advanced synthesis and catalysis, 361(12), 2936-2944 (2019)
Synthesis and structure-activity relationships of biarylcarboxamide bis-aminopyrrolidine urea derived small-molecule antagonists of the melanin-concentrating hormone receptor-1 (MCH-R1)
Rowbottom MW, et al.
Bioorganic & medicinal chemistry letters, 15(14), 3439-3445 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service