430676
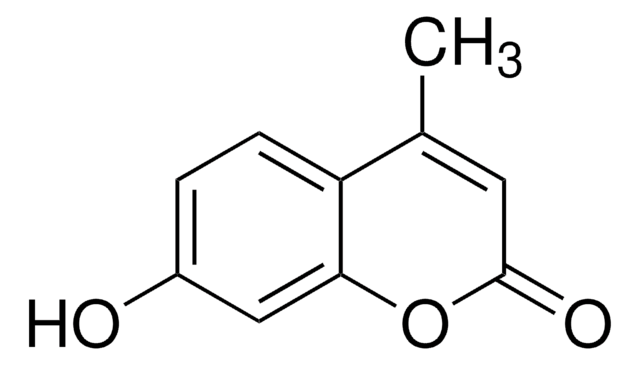
2,2,5,7,8-Pentamethyl-6-chromanol
97%
Synonym(s):
α-C-1-Chromanol, 2,2,5,7,8-Pentamethyl-3,4-dihydrochromen-6-ol, 2,2,5,7,8-Pentamethyl-6-hydroxychroman, 3,4-Dihydro-2,2,5,7,8-pentamethyl-2H-1-benzopyran-6-ol, 6-Hydroxy-2,2,5,7,8-pentamethylchroman
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C14H20O2
CAS Number:
Molecular Weight:
220.31
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
General description
2,2,5,7,8-Pentamethyl-6-chromanol has been reported as an vitamin E model compound. It undergoes oxidation in presence of various alcohols, ranging from methanol to cholesterol, affords 5-alkoxymethyl-2,2,7,8-tetramethyl-6-chromanols. Antioxidant moiety of vitamin E, 2,2,5,7,8-pentamethyl-6-chromanol (PMCol), is reported to have antiandrogen activity in prostate carcinoma cells.
Application
2,2,5,7,8-Pentamethyl-6-chromanol is the suitable reagent used for the quantitative analysis of α-tocopherol by plasma-based gas chromatography/tandem mass spectrometry (GC/MS/MS) using a tabletop quadrupole ion trap mass spectrometer. It may be employed as α-tocopherol model compound and on oxidation by t-butyl hydroperoxide in chloroform, in the presence of alcohol, affords 5-alkoxymethyl-2,2,7,8-tetramethyl-6-chromanol. It may be used as internal standard for the determination of α- and γ-tocopherol in the rabbit serum and liver by HPLC.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Qing Jiang et al.
Proceedings of the National Academy of Sciences of the United States of America, 105(51), 20464-20469 (2008-12-17)
Cyclooxygenase (COX-1/COX-2)-catalyzed eicosanoid formation plays a key role in inflammation-associated diseases. Natural forms of vitamin E are recently shown to be metabolized to long-chain carboxychromanols and their sulfated counterparts. Here we find that vitamin E forms differentially inhibit COX-2-catalyzed prostaglandin
Urs Hengartner et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(4), 1306-1311 (2009-12-17)
Alpha-tocopherol was synthesized from a chiral intermediate alpha-hydroxy ester by means of two ring-closing methods to yield the chromanol in 94% diastereomeric excess.
C Suarna et al.
Lipids, 24(1), 56-60 (1989-01-01)
The vitamin E model compound, 2,2,5,7,8-pentamethyl-6-chromanol, has been oxidized with t-butyl hydroperoxide in chloroform in order to simulate in vivo oxidations due to lipid hydroperoxides. In the presence of a variety of alcohols, ranging from methanol to cholesterol, the corresponding
New oxidation products of 2, 2, 5, 7, 8-pentamethyl-6-chromanol.
Suarna C, et al.
Lipids, 23(12), 1129-1131 (1988)
Todd A Thompson et al.
Molecular cancer therapeutics, 2(8), 797-803 (2003-08-27)
Antioxidants, such as vitamin E, are being investigated for efficacy in prostate cancer prevention. In this study, we show that the antioxidant moiety of vitamin E, 2,2,5,7,8-pentamethyl-6-chromanol (PMCol), has antiandrogen activity in prostate carcinoma cells. In the presence of PMCol
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service