All Photos(1)
About This Item
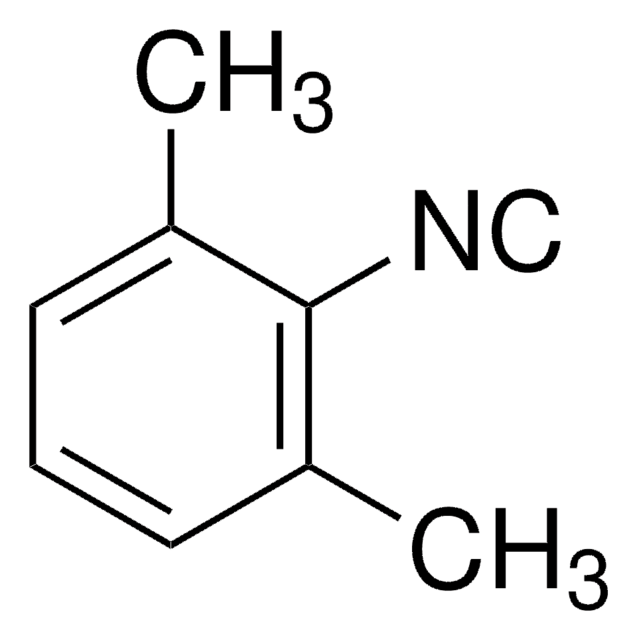
Empirical Formula (Hill Notation):
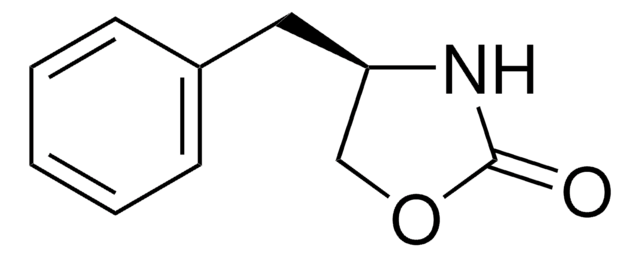
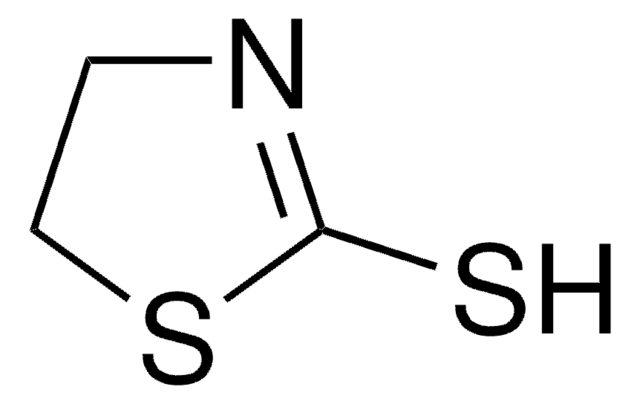
C10H11NS2
CAS Number:
Molecular Weight:
209.33
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0%
optical activity
[α]20/D +122±5°, c = 1% in chloroform
optical purity
enantiomeric ratio: ≥99:1 (LC)
SMILES string
S=C1N[C@@H](CS1)Cc2ccccc2
InChI
1S/C10H11NS2/c12-10-11-9(7-13-10)6-8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H,11,12)/t9-/m1/s1
InChI key
SLDUGQISGRPGAW-SECBINFHSA-N
Application
A highly selective and efficient chiral auxiliary which can be directly reduced to its corresponding aldehyde and the chiral auxiliary by reductive cleavage with diisobutylaluminum hydride.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Velazquez. F.; Olivo, H. F.
Current Organic Chemistry, 6, 303-303 (2002)
M T Crimmins et al.
Organic letters, 2(6), 775-777 (2001-02-07)
[formula: see text] Asymmetric aldol additions using chlorotitanium enolates of thiazolidinethione propionates proceed with high diastereoselectivity for the "Evans" or "non-Evans" syn product depending on the nature and amount of the base used. With (-)-sparteine as the base, selectivities of
Articles
The asymmetric aldol reaction mediated by chiral auxiliaries is considered to be one of the most important methods for asymmetric C-C bond formation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service