427764
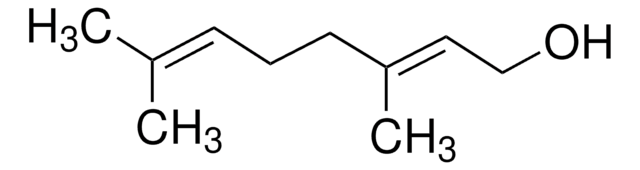
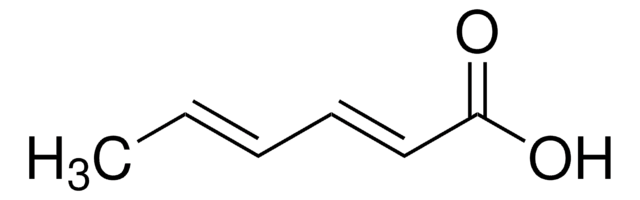
Geranic acid
technical grade, 85%
Synonym(s):
3,7-Dimethyl-2,6-octadienoic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2C=CHCH2CH2C(CH3)=CHCO2H
CAS Number:
Molecular Weight:
168.23
Beilstein:
1763804
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
85%
form
liquid
refractive index
n20/D 1.484 (lit.)
bp
250 °C (lit.)
density
0.97 g/mL at 25 °C (lit.)
SMILES string
C\C(C)=C\CC\C(C)=C\C(O)=O
InChI
1S/C10H16O2/c1-8(2)5-4-6-9(3)7-10(11)12/h5,7H,4,6H2,1-3H3,(H,11,12)/b9-7+
InChI key
ZHYZQXUYZJNEHD-VQHVLOKHSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Geranic acid is a polyunsaturated fatty acid. It is used as a bio-friendly cross-linker in the fabrication of a molecularly imprinted polymer. Geranic acid belongs to the terpenoid family. It exists as two stereoisomers with trans and cis geometry across the conjugated double bond.
Application
- Enhancement of antibiotic properties: Capric acid and geranic acid were used to improve the pharmaceutical properties and antibacterial activity of levofloxacin (Alkhawaja et al., 2023).
- Aryl hydrocarbon receptor modulation: Research on natural deep eutectic solvents including fatty acids like geranic acid has demonstrated their capability to modulate the aryl hydrocarbon receptor independently of traditional ligands, suggesting potential in metabolic regulation and therapeutic applications (Denis et al., 2023).
- Transdermal delivery systems: A study on ionic liquids composed of geranic acid for obesity treatment highlighted their use in transdermal drug delivery systems, presenting a non-invasive alternative for therapeutic management (Lu et al., 2023).
- Antimicrobial and antielastase properties: Geranic acid has been shown to inhibit elastase activity and bacterial growth, offering potential applications in oral health products and treatments for conditions involving pathogenic bacteria and inflammation (Laird et al., 2023).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
271.4 °F - closed cup
Flash Point(C)
133 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Monitoring tea fermentation/manufacturing by direct analysis in real time (DART) mass spectrometry.
Fraser K, et al.
Food Chemistry, 141(3), 2060-2065 (2013)
U Heyen et al.
Applied and environmental microbiology, 66(7), 3004-3009 (2000-07-06)
Monoterpenes with an unsaturated hydrocarbon structure are mineralized anaerobically by the denitrifying beta-proteobacterium Alcaligenes defragrans. Organic acids occurring in cells of A. defragrans and culture medium were characterized to identify potential products of the monoterpene activation reaction. Geranic acid (E,E-3,7-dimethyl-2,6-octadienoic
D C Alexander et al.
Journal of bacteriology, 176(22), 7079-7084 (1994-11-01)
We report that rfe mutants of wild-type strains of Escherichia coli O7, O18, O75, and O111 did not express O-specific polysaccharide unless the rfe mutation was complemented by a cloned rfe gene supplied in a plasmid. The O polysaccharides in
Tanaya Chatterjee
Biotechnology and applied biochemistry, 39(Pt 3), 303-306 (2004-05-25)
Microbial degradation of geraniol, a natural monoterpene alcohol, was studied using a Rhodococcus sp. strain GR3 isolated from soil. The bioconversion product was identified as geranic acid [(2 E )-3,7-dimethylocta-2,6-dienoic acid] and its structure was established by (1)H-NMR, Fourier-transform IR
On the constituents of a Chinese rose oil.
Ohno Y and Tanaka S.
Agricultural and Biological Chemistry, 41(2), 399-401 (1977)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service