424447
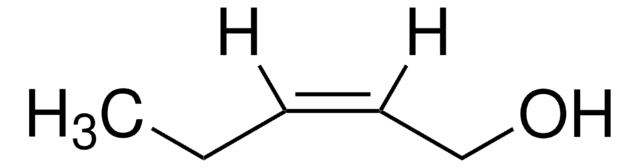
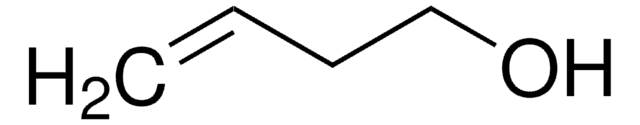
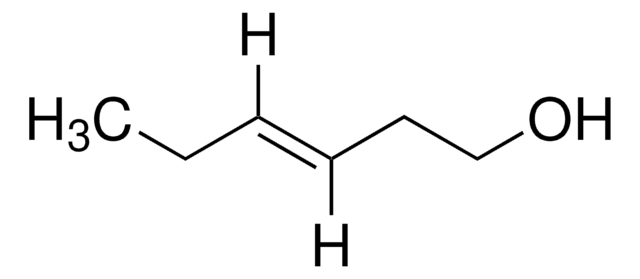
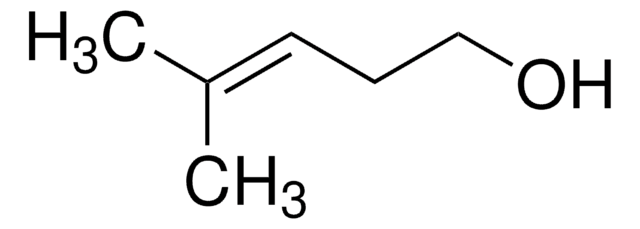
trans-2-Penten-1-ol
95%
Synonym(s):
(2E)-2-Penten-1-ol, (E)-Pent-2-en-1-ol, trans-2-Pentenol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C2H5CH=CHCH2OH
CAS Number:
Molecular Weight:
86.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
liquid
refractive index
n20/D 1.434 (lit.)
bp
139-139.5 °C (lit.)
density
0.847 g/mL at 25 °C (lit.)
SMILES string
CC\C=C\CO
InChI
1S/C5H10O/c1-2-3-4-5-6/h3-4,6H,2,5H2,1H3/b4-3+
InChI key
BTSIZIIPFNVMHF-ONEGZZNKSA-N
General description
trans-2-Penten-1-ol is an allyl alcohol. It is one of the volatile compounds found in olive oil, cashew apple juice and fermented cucumber brines. The rate constants and product ion distributions of its reaction with H3O+, NO+ and O2.+ ions have been studied using selected ion flow tube (SIFT).
Application
trans-2-Penten-1-ol may be used in the synthesis of the following:
- leustroducsin B
- trichloroacetimidate
- (E)-2,3,3′-trifluoro-4-(2-(trans-4-pentylcyclohexyl)ethyl)-4′-(pent-2-enyloxy)biphenyl
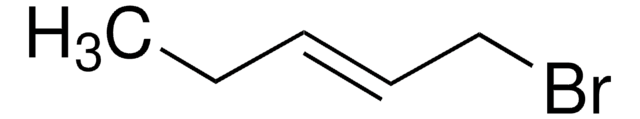
- trans-1-bromo-2-pentene
- trans-1-chloro-2-pentene
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
118.4 °F - closed cup
Flash Point(C)
48 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kazuyuki Miyashita et al.
The Journal of organic chemistry, 73(14), 5360-5370 (2008-06-14)
Leustroducsin B was synthesized via a convergent route based on division of the leustroducsin molecule into three segments A, B, and C. Two coupling reactions (Julia coupling reaction and Nozaki-Hiyama-Kishi (NHK) reaction) were employed for coupling of segments A and
Suzanne D Johanningsmeier et al.
Journal of food science, 76(1), C168-C177 (2011-05-04)
A nontargeted, comprehensive 2-dimensional gas chromatography-time-of-flight mass spectrometry (GC×GC-TOFMS) method was developed for the analysis of fermented cucumber volatiles before and after anaerobic spoilage. Volatile compounds extracted by solid-phase microextraction were separated on a polyethylene glycol 1st-dimension column and 14%
Biogeneration of volatile compounds in virgin olive oil: their evolution in relation to malaxation time.
Angerosa F, et al.
Journal of Agricultural and Food Chemistry, 46(8), 2940-2944 (1998)
A selected ion flow tube study of the reactions of H3O+, NO+ and O2.+ with a series of C5, C6 and C8 unsaturated biogenic alcohols.
Schoon N, et al.
International Journal of Mass Spectrometry, 263(2-3), 127-136 (2007)
Stereoregulated synthesis of unsaturated compounds Communication 9. Stereochemistry of the reactions of aldehydes with ?, ?-unsaturated triphenylphosphonium ylides [alkylidenetriphenylphosphoranes].
Bergel'son LD, et al.
Bulletin of the Academy of Sciences of the USSR, Division of chemical science, 15(3), 468-473 (1966)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service