All Photos(1)
About This Item
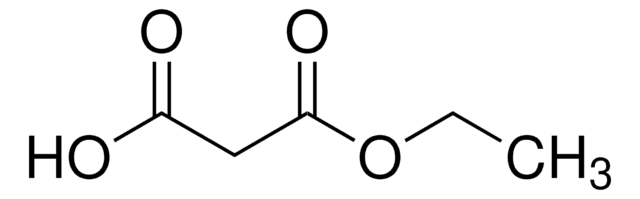
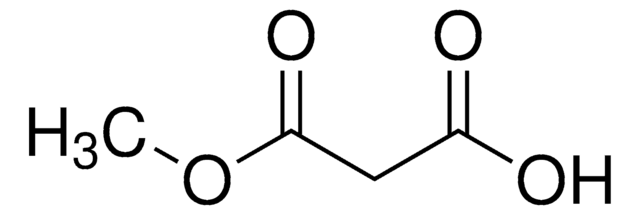
Linear Formula:
(CH3)3COCOCH2COOH
CAS Number:
Molecular Weight:
160.17
Beilstein:
1765457
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
impurities
5% methanesulfonyl chloride
refractive index
n20/D 1.426 (lit.)
bp
90 °C/2 mmHg (lit.)
mp
19-20 °C (lit.)
density
1.04 g/mL at 25 °C (lit.)
SMILES string
CC(C)(C)OC(=O)CC(O)=O
InChI
1S/C7H12O4/c1-7(2,3)11-6(10)4-5(8)9/h4H2,1-3H3,(H,8,9)
InChI key
NGGGZUAEOKRHMA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Mono-tert-Butyl malonates is an ester. It is reported to be an aminoacylase inhibitor. Preparation of mono-tert-Butyl malonates has been described.
Application
Mono-tert-Butyl malonates may be used in the preparation of the following:
- dendritic precursor to asymmetric methanofullerenes
- hapten-3,6-(O,S-dimethylthiophosphoramido)-6-oxohexanoic acid
- hapten-4,3-(O,S-dimethylthiophosphoramido)-3-oxopropanoic acid
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Inhalation - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
195.8 °F - closed cup
Flash Point(C)
91 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Helen S Toogood et al.
Extremophiles : life under extreme conditions, 6(2), 111-122 (2002-05-16)
A thermostable L-aminoacylase from Thermococcus litoralis was cloned, sequenced, and overexpressed in Escherichia coli. The enzyme is a homotetramer of 43 kDa monomers and has an 82% sequence identity to an aminoacylase from Pyrococcus horikoshii and 45% sequence identity to
Formation of high-aspect-ratio helical nanorods via chiral self-assembly of fullerodendrimers.
Hilmer AJ, et al.
The Journal of Physical Chemistry Letters, 5(5), 929-934 (2014)
Facile Preparation and Purification of Mono tert-Butyl Malonate.
Tararov VI, et al.
Synthetic Communications, 36(2), 187-191 (2006)
Jae Koo Lee et al.
Journal of agricultural and food chemistry, 51(13), 3695-3703 (2003-06-12)
A competitive indirect enzyme-linked immunosorbent assay (ciELISA) for the organophosphorus insecticide acephate, O,S-dimethyl acetylphosphoramidothioate, was developed using a polyclonal antibody. Five different haptens mimicking the analyte were synthesized and conjugated with the carrier proteins bovine serum albumin (BSA) and keyhole
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service