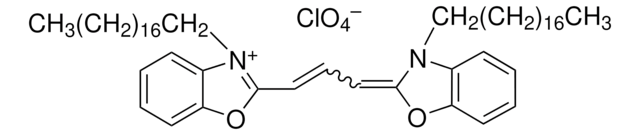
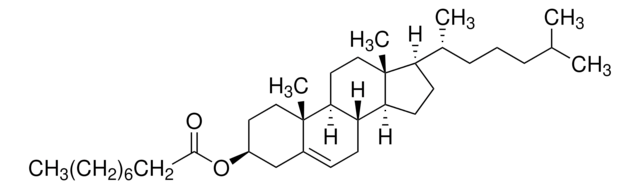
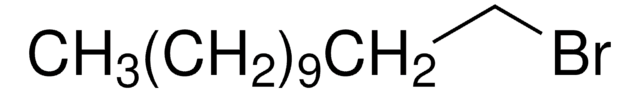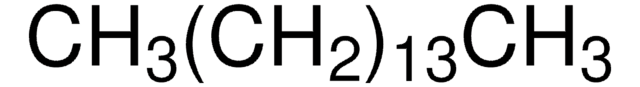410497
4-(Dicyanomethylene)-2-methyl-6-(4-dimethylaminostyryl)-4H-pyran
Dye content 98 %
Synonym(s):
DCM
About This Item
Recommended Products
form
solid
Quality Level
composition
Dye content, 98%
mp
215-220 °C (lit.)
λmax
468 nm
OLED Device Performance
ITO/Alq3:DCM/Alq3/Mg:Ag
ITO/TPD/Alq3:DCM (10%)/Alq3/Mg:Ag
SMILES string
CN(C)c1ccc(\C=C\C2=CC(\C=C(C)O2)=C(\C#N)C#N)cc1
InChI
1S/C19H17N3O/c1-14-10-16(17(12-20)13-21)11-19(23-14)9-6-15-4-7-18(8-5-15)22(2)3/h4-11H,1-3H3/b9-6+
InChI key
YLYPIBBGWLKELC-RMKNXTFCSA-N
General description
Application
Features and Benefits
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Eye Irrit. 2 - Flam. Sol. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
109.4 °F - closed cup
Flash Point(C)
43 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
A tutorial of Display and Optoelectronics from Sigma-Aldrich.
Developed in the last several years, fluorescence quenching microscopy (FQM) has enabled rapid, inexpensive, and high-fidelity visualization of two-dimensional (2D) materials such as graphene-based sheets and MoS2.
Graphene has emerged as the new wonder material. Being only one atom thick and composed of carbon atoms arranged in a hexagonal honeycomb lattice structure, the interest in this material has exploded exponentially since 2004 when it was first isolated and identified using a very simple method.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/209/681/63a4048f-a2a7-496b-814d-ccb4b5b76124/640/63a4048f-a2a7-496b-814d-ccb4b5b76124.png)