402168
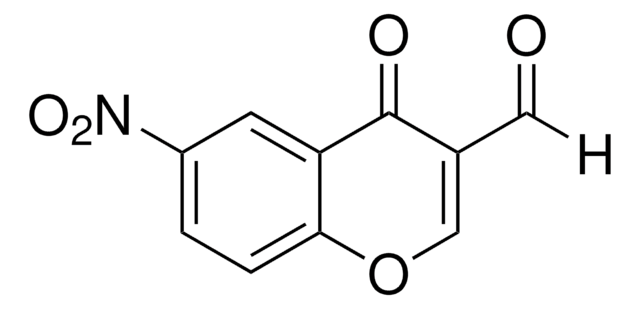
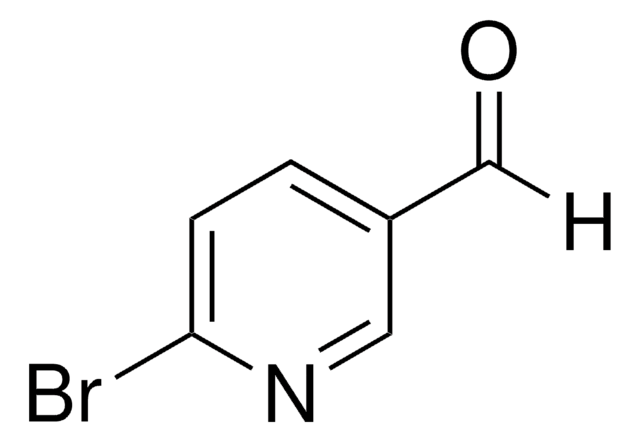
6-Bromo-3-formylchromone
99%
Synonym(s):
6-Bromo-4-oxo-4H-1-benzopyran-3-carboxaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H5BrO3
CAS Number:
Molecular Weight:
253.05
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
190-193 °C (lit.)
SMILES string
[H]C(=O)C1=COc2ccc(Br)cc2C1=O
InChI
1S/C10H5BrO3/c11-7-1-2-9-8(3-7)10(13)6(4-12)5-14-9/h1-5H
InChI key
PCEZXSJBHMOQFT-UHFFFAOYSA-N
Gene Information
human ... PTPN1(5770)
General description
6-Bromo-3-formylchromone (6-Bromo-4-oxo-4H-1-benzopyran-3-carboxaldehyde) is a 3-formylchromone derivative. In vivo cytotoxic activity of 6-bromo-3-formylchromone against normal and tumor cells has been tested.
Application
6-Bromo-3-formylchromone is the suitable reagent used in a study to investigate the multidrug resistance reversal by some 3- formylchromones in human colon cancer and mouse lymphoma cells transfected with the human MDR1 gene. It may be used in the preparation of chromone containing sulfonamides.
6-Bromo-3-formylchromone may be used in the preparation of 6′-bromopyranothiazine-4,7-diones.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A study of the reactions of 2-aryl-4-hydroxy-6H-1,3-thiazin-6-ones with chromone-3-carboxaldehydes.
Shutov RV, et al.
Tetrahedron Letters, 52(2), 266-269 (2011)
Mariya al-Rashida et al.
Bioorganic & medicinal chemistry, 19(11), 3367-3371 (2011-05-11)
Series of chromone containing sulfonamides were prepared by the reaction of (un)substituted 3-formylchromones with 3-aminobenzenesulfonamide and 4-aminobenzenesulfonamide. Bovine carbonic anhydrase (bCA) inhibitory activity of these newly synthesized compounds was determined. All compounds were active and possessed excellent bCA inhibitory activities
Masami Kawase et al.
In vivo (Athens, Greece), 21(5), 829-834 (2007-11-21)
Several 3-formylchromone derivatives were examined for their tumor cell-cytotoxic, anti-Helicobacter pylori, urease inhibitory and anti-HIV activity. Comparing their relative cytotoxicity against four human tumor cell lines and three normal human cells, tumor cell-specific cytotoxicity was detected in some 3-formylchromone derivatives.
Zoltán Baráth et al.
In vivo (Athens, Greece), 20(5), 645-649 (2006-11-10)
Several new 3-formylchromone derivatives proved to be modifiers of multidrug resistance in mouse lymphoma cells and in human Colo320 colon cancer cells. There is apparently a structure-activity relationship between the antiproliferative multidrug resistance-reversing effect and the chemical structure of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service