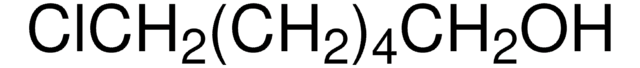All Photos(1)
About This Item
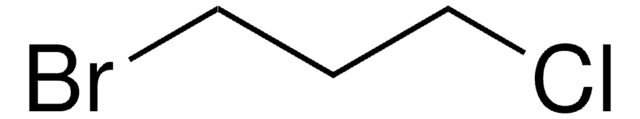
Linear Formula:
I(CH2)4Cl
CAS Number:
Molecular Weight:
218.46
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
contains
copper as stabilizer
refractive index
n20/D 1.54 (lit.)
bp
88-89 °C/19 mmHg (lit.)
density
1.785 g/mL at 25 °C (lit.)
SMILES string
ClCCCCI
InChI
1S/C4H8ClI/c5-3-1-2-4-6/h1-4H2
InChI key
JXOSPTBRSOYXGC-UHFFFAOYSA-N
General description
1-Chloro-4-iodobutane is a halogenated hydrocarbon. It is an α,ω-dihaloalkane and undergoes electrogenerated Nickel(I) salen (N,N′-bis(salicylidene)ethylenediamine) catalyzed reduction to afford 1,8-dichlorooctane. Electrochemical reduction of 1-chloro-4-iodobutane at glassy carbon cathode has been investigated by cyclic voltammetry and controlled-potential electrolysis.
Application
1-Chloro-4-iodobutane may be used in the following studies:
- Preparation of 6-hendecenoic acid.
- Catalytic asymmetric synthesis of levobupivacaine.
- Synthesis of alkaloids such as deoxyvasicinone, mackinazolinone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
199.4 °F - closed cup
Flash Point(C)
93 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Studies directed towards asymmetric synthesis of levobupivacaine.
Kumar S and Ramachandran U.
Tetrahedron Letters, 46(1), 19-21 (2005)
The synthesis of unsaturated fatty acids.
K AHMAD et al.
Journal of the American Chemical Society, 70(5), 1699-1699 (1948-05-01)
W Russell Bowman et al.
Organic & biomolecular chemistry, 5(1), 103-113 (2006-12-14)
Alkyl, aryl, heteroaryl and acyl radicals have been cyclised onto the 2-position of 3H-quinazolin-4-one. The side chains containing the radical precursors were attached to the nitrogen atom in the 3-position. The cyclisations take place by aromatic homolytic substitution hence retain
Electrochemical reduction of 1, 4-dihalobutanes at carbon cathodes in dimethylformamide.
Pritts WA and Peters DG.
Journal of Electroanalytical Chemistry, 380(1), 147-160 (1995)
Keivan Sadrerafi et al.
Drug design, development and therapy, 12, 987-995 (2018-05-08)
Our previous study indicated that carborane containing small-molecule 1-(hydroxymethyl)-7-(4'-(trans-3″-(3'″-pyridyl)acrylamido)butyl)-1,7-dicarbadodecaborane (hm-MC4-PPEA), was a potent inhibitor of nicotinamide phosphoribosyltransferase (Nampt). Nampt has been shown to be upregulated in most cancers and is a promising target for the treatment of many different types
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service