392189
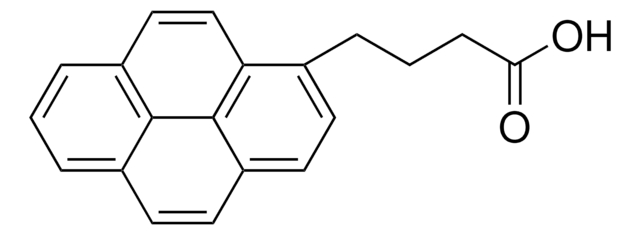
1-Pyreneacetic acid
97%
Synonym(s):
(1-Pyrenyl)acetic acid
About This Item
Recommended Products
Quality Level
Assay
97%
form
solid
mp
210-212 °C (dec.) (lit.)
SMILES string
OC(=O)Cc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C18H12O2/c19-16(20)10-14-7-6-13-5-4-11-2-1-3-12-8-9-15(14)18(13)17(11)12/h1-9H,10H2,(H,19,20)
InChI key
SDJCLYBBPUHKCD-UHFFFAOYSA-N
General description
Application
- Synthesis of N-(2-(methylthio)ethyl)-2-(pyren-1-yl)acetamide, a pyrene amide based Pd2+ probe.[4]
- Synthesis of pyrene-modified β-cyclodextrin.[5]
- To functionalize single walled carbon nanotube field effect transistors (CNT FETs).[6]
- As an agent for characterizing grafting degrees and reactivity of the ester functionalized polypropylenes.[7]
- Synthesis sawhorse-type diruthenium tetracarbonyl complexes.[8]
- Synthesis of (±)-2-(1-pyrenyl)propionic acid, a chiral carboxylic acid.[9]
- Reversible noncovalent functionalization of single walled carbon nanotubes (SWNTs).[10]
- Preparation of peptide nucleic acid (PNA) probes.[11]
- As an internal reference compound in the assessment of solid phase reaction by HPLC-UV.[12]
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service