390836
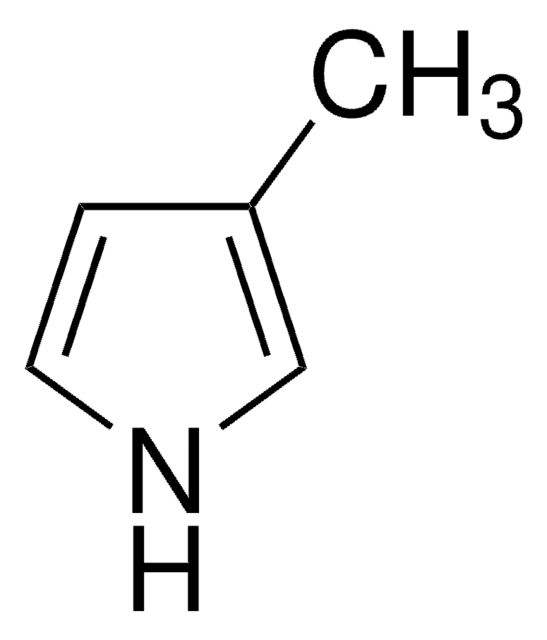
2,4-Dimethylpyrrole
97%
Synonym(s):
2,4-Dimethyl-1-pyrrole, 2,4-Dimethyl-1H-pyrrole, 2,4-Dimethyl-3H-pyrrole
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
Empirical Formula (Hill Notation):
C6H9N
CAS Number:
Molecular Weight:
95.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.496 (lit.)
bp
165-167 °C (lit.)
density
0.924 g/mL at 25 °C (lit.)
SMILES string
Cc1c[nH]c(C)c1
InChI
1S/C6H9N/c1-5-3-6(2)7-4-5/h3-4,7H,1-2H3
InChI key
MFFMQGGZCLEMCI-UHFFFAOYSA-N
Related Categories
General description
2,4-Dimethylpyrrole is a substituted pyrrole. Its synthesis by using ethyl acetoacetate as starting material has been reported. Its basicity has been evaluated from UV spectral data. Its photodecomposition on irradiation has been reported to afford H2, CH4, C2H6 and polymeric products.
Application
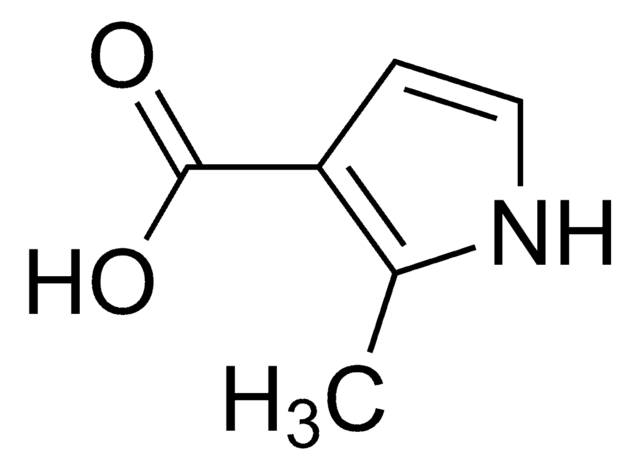
2,4-Dimethylpyrrole may be used in the synthesis of the following:
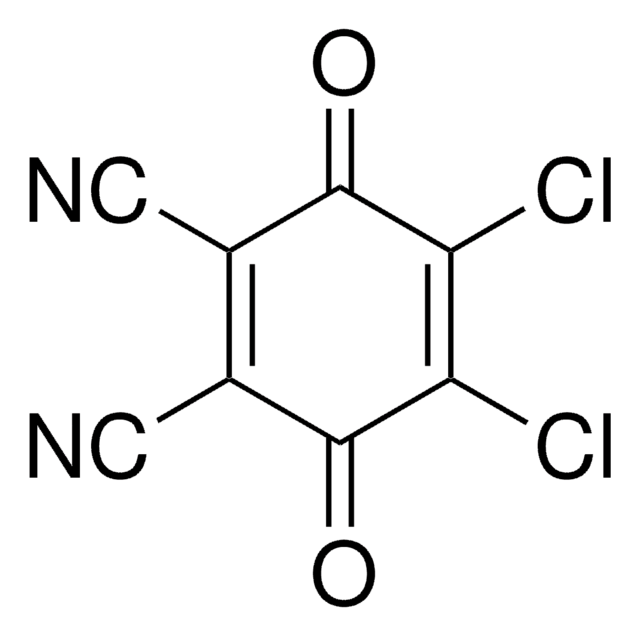
- 2,5-bis(2′,4′-dimethyl-5′-pyrryl)p-benzoquinone
- boron dipyrromethene (BODIPY) dyes
- 2,4-dimethyl-6-methoxyprodigiosene
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Photolysis of 2, 5-and 2, 4-dimethylpyrrole vapors at room temperature.
Wu EC and Heicklen J.
Canadian Journal of Chemistry, 50(11), 1678-1689 (1972)
Addition of pyrroles to 1, 4-benzoquinone.
Bullock, E.
Canadian Journal of Chemistry, 36(12), 1744-1744 (1958)
Seda Çetindere et al.
Journal of fluorescence, 29(5), 1143-1152 (2019-08-14)
In the present work, novel water-soluble cyclotriphosphazene derivatives (3b and 4b) were synthesized by 'click' reactions between cyclotriphosphazene derivative with hydrophilic glycol side groups (2) and Bodipy's (3a and 4a). All newly synthesized compounds (2, 3b and 4b) were characterized
Thermal decomposition and ring expansion in 2, 4-dimethylpyrrole. Single pulse shock tube and modeling studies.
Lifshitz A, et al.
The Journal of Physical Chemistry A, 107(24), 4851-4861 (2003)
Reactions of the Perfluoroalkylnitriles. VIII. Syntheses of 1, 3, 5-Triazines with Specific Groups in the 2, 4, or 6 Positions.
Brown H, et al.
The Journal of Organic Chemistry, 32(1), 231-233 (1967)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service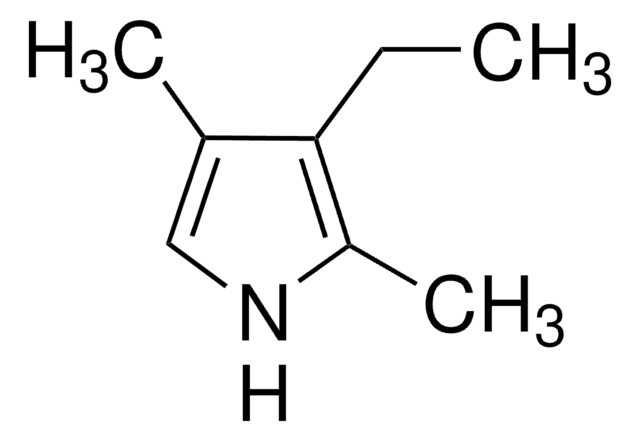




![Difluoro{2-[(3,5-dimethyl-2H-pyrrol-2-ylidene-N)methyl]-3,5-dimethyl-1H-pyrrolato-N}boron 99% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/518/861/c19c64be-654e-472e-a069-30ffccb1a8cd/640/c19c64be-654e-472e-a069-30ffccb1a8cd.png)