381454
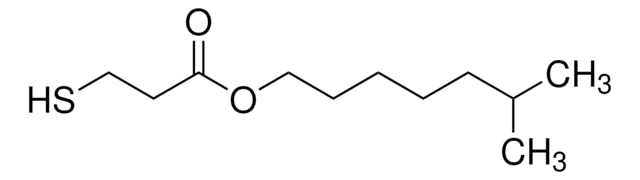
Butyl 3-mercaptopropionate
98%
Synonym(s):
Butyl 3-mercaptopropanoate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HSCH2CH2CO2(CH2)3CH3
CAS Number:
Molecular Weight:
162.25
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.457 (lit.)
bp
101 °C/12 mmHg (lit.)
density
0.999 g/mL at 25 °C (lit.)
SMILES string
CCCCOC(=O)CCS
InChI
1S/C7H14O2S/c1-2-3-5-9-7(8)4-6-10/h10H,2-6H2,1H3
InChI key
MGFFVSDRCRVHLC-UHFFFAOYSA-N
General description
Butyl 3-mercaptopropionate (3MPA) is a monofunctional thiol that can be used as a cross-linker and chain transferring agent for controlling the molecular weight of the polymer. It can be used in the thiolene photopolymerization.
Application
3MPA can be used as a ligand which can functionalize the quantum dots for the development of high luminescence light emitting diodes. It can also be used as a crosslinking monomeric unit for the preparation of thiol-acrylate based photopolymers.
Used as a reactant for thiol-yne photopolymerizations to form highly-cross-linked networks.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
199.4 °F - closed cup
Flash Point(C)
93 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Thiol- Yne photopolymerizations: novel mechanism, kinetics, and step-growth formation of highly cross-linked networks
Fairbanks BD, et al.
Macromolecules, 42(1), 211-217 (2008)
Development of a poly (methyl methacrylate-co-n-butyl methacrylate) copolymer binder system
Vail NK, et al.
Journal of Applied Polymer Science, 52(6), 789-812 (1994)
Benjamin D Fairbanks et al.
Macromolecules, 42(1), 211-217 (2009-05-23)
Radical-mediated thiol-yne step-growth photopolymerizations are utilized to form highly cross-linked polymer networks. This reaction mechanism is shown to be analogous to the thiol-ene photopolymerization; however, each alkyne functional group is capable of consecutive reaction with two thiol functional groups. The
High luminescence efficiency white light emitting diodes based on surface functionalized quantum dots dispersed in polymer matrices
Yoon C, et al.
Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 428(22), 86-91 (2013)
Initiation and kinetics of thiol-ene photopolymerizations without photoinitiators
Cramer NB, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 42(22), 5817-5826 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)