379387
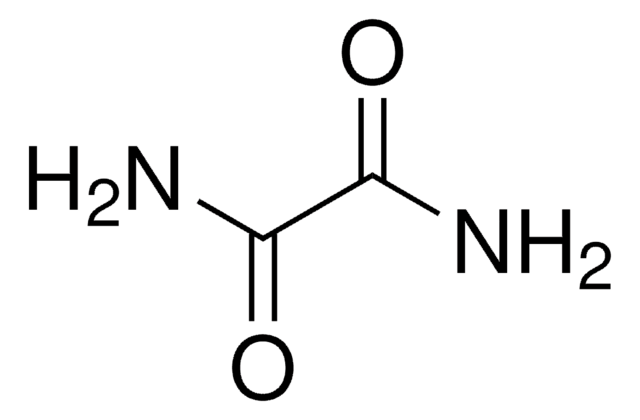
Dithiooxamide
97%
Synonym(s):
Dithiooxalic diamide, Rubeanic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2CSCSNH2
CAS Number:
Molecular Weight:
120.20
Beilstein:
605577
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
≥300 °C (lit.)
solubility
ethanol: soluble 40 mg/10 mL, clear, red
SMILES string
NC(=S)C(N)=S
InChI
1S/C2H4N2S2/c3-1(5)2(4)6/h(H2,3,5)(H2,4,6)
InChI key
OAEGRYMCJYIXQT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Dithiooxamide is reported to form complexes with Ni(II).
Application
Dithiooxamide may be used in the following studies:
- Synthesis of thiazolothiazole-linked porous organic polymers under solvothermal conditions.
- As modifier to prepare the modified glassy carbon electrode, used to investigate the electrochemical properties of quercetin, an important flavonoid derivative.
- Synthesis of new chelating resin of dithiooxamide (rubeanic acid)-formaldehyde (DTOF), used in separation and concentration of silver ions.
- Synthesis of N,N′-disubstituted dithiooxamides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xiang Zhu et al.
Chemical communications (Cambridge, England), 50(95), 15055-15058 (2014-10-21)
Thiazolothiazole-linked porous organic polymers have been synthesized from a facile catalyst-free condensation reaction between aldehydes and dithiooxamide under solvothermal conditions. The resultant porous frameworks exhibit a highly selective uptake of CO2 over N2 under ambient conditions.
Preparation of Dithiooxamide Derivatives.
Hurd RN, et al.
The Journal of Organic Chemistry, 26(10), 3980-3987 (1961)
Nickel (II) complexes with dithiooxamide, N, N'-di-methyl-and N, N'-di-hydroxyethyl-dithiooxamide.
Peyronel G, et al.
Inorgorganica Chimica Acta, 5, 627-633 (1971)
Ayşen Demir Mülazımoğlu et al.
Sensors (Basel, Switzerland), 12(4), 3916-3928 (2012-06-06)
Electrochemical oxidation of quercetin, as an important biological molecule, has been studied in non-aqueous media using cyclic voltammetry, electrochemical impedance spectroscopy and scanning electron microscopy. To investigate the electrochemical properties of quercetin, an important flavonoid derivative, on a different surface
Zeliyha Celik et al.
Journal of hazardous materials, 174(1-3), 556-562 (2009-10-13)
In this study, a new chelating resin of dithiooxamide (rubeanic acid)-formaldehyde (DTOF) has been synthesized by the reaction of dithiooxamide and formaldehyde. Also a well-known chelating resin of thiourea (thiooxamide)-formaldehyde (TUF) has been prepared by the reaction of thiourea and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service