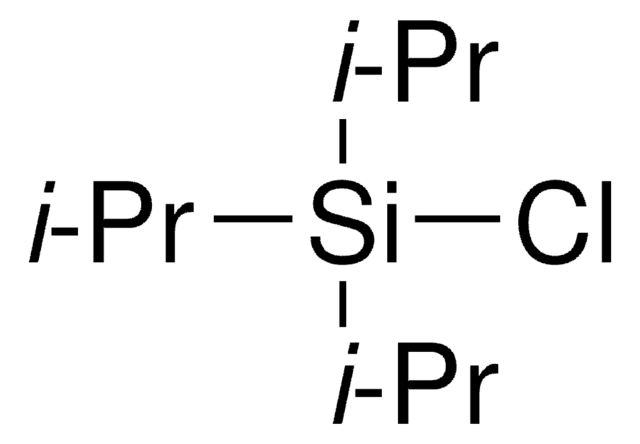All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H25NSi
CAS Number:
Molecular Weight:
223.43
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.492 (lit.)
bp
78 °C/0.4 mmHg (lit.)
density
0.904 g/mL at 25 °C (lit.)
SMILES string
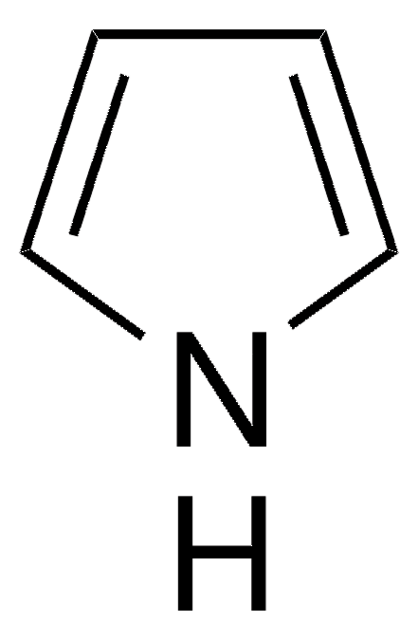
CC(C)[Si](C(C)C)(C(C)C)n1cccc1
InChI
1S/C13H25NSi/c1-11(2)15(12(3)4,13(5)6)14-9-7-8-10-14/h7-13H,1-6H3
InChI key
FBQURXLBJJNDBX-UHFFFAOYSA-N
General description
1-(Triisopropylsilyl)pyrrole (TISP), a heterocyclic building block, is a pyrrole derivative. TISP has been reported to generate pyrrolic cation radicals during cyclovoltammetric studies, via electroreduction. It participates in various electrophilic substitution reactions specifically at β-position, via reaction with various electrophilic reagents (Br+, I+,NO2+,etc).
Application
1-(Triisopropylsilyl)pyrrole may be employed as reagent in perfluoroalkylation and Vilsmeier formylation reactions. It may be used in the preparation of:
- ethyl 2-(2,4-dinitrophenylhydrazono]-3-[ 1-(triisopropylsily1)-pyrrol-2-yflpropanoate
- heterocyclic base, 3-nitropyrrole
- 3-nitropyrrole, required for the synthesis of 1 -(2′-deoxy-β-D-ribofuranosyl)-3-nitropyrrole
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
224.6 °F - closed cup
Flash Point(C)
107 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
N-(triisopropylsilyl) pyrrole. A progenitor" par excellence" of 3-substituted pyrroles.
Bray BL, et al.
The Journal of Organic Chemistry, 55(26), 6317-6328 (1990)
Daniel A Harki et al.
Biochemistry, 41(29), 9026-9033 (2002-07-18)
Synthetic small molecules that promote viral mutagenesis represent a promising new class of antiviral therapeutics. Ribavirin is a broad-spectrum antiviral nucleoside whose antiviral mechanism against RNA viruses likely reflects the ability of this compound to introduce mutations into the viral
Observation of the cation radicals of pyrrole and of some substituted pyrroles in fast-scan cyclic voltammetry. Standard potentials and lifetimes.
Andrieux CP, et al.
Journal of the American Chemical Society, 112(6), 2439-2440 (1990)
Reaction of pyrroles with ethyl 2-nitroso-and 2-azo-propenoates, and with ethyl cyanoformate N-oxide: a comparison of the reaction pathways.
Gilchrist TL and Lemos A.
Journal of the Chemical Society. Perkin Transactions 1, 13, 1391-1395 (1993)
Synthesis, Structure, and Deoxyribonucleic Acid Sequencing with a Universal Nucleoside: 1-(2'-Deoxy-. beta.-D-ribofuranosyl)-3-nitropyrrole.
Bergstrom DE, et al.
Journal of the American Chemical Society, 117(4), Synthesis-Synthesis (1999)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service