All Photos(3)
About This Item
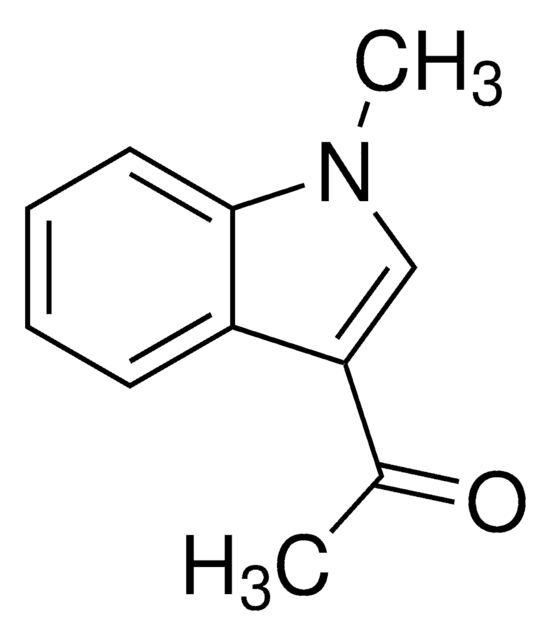
Empirical Formula (Hill Notation):
C10H9NO
CAS Number:
Molecular Weight:
159.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.607 (lit.)
bp
123-125 °C/8 mmHg (lit.)
density
1.387 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(=O)n1ccc2ccccc12
InChI
1S/C10H9NO/c1-8(12)11-7-6-9-4-2-3-5-10(9)11/h2-7H,1H3
InChI key
UUCUQJHYUPXDHN-UHFFFAOYSA-N
General description
Quantum chemical calculations of ground state energy, geometrical structure and vibrational wavenumbers of 1-acetylindole has been carried out using density functional (DFT/B3LYP) method. Regioselective acylations of 1-acetylindole (N-acetylindole) under Friedel-Crafts reaction has been reported. Reaction of 1-acetylindole with manganese(III) acetate in the presence of malonic acid, is reported to afford 4-acetyl-3,3a,4,8b-tetrahydro-2H-furo[3,2-b]indol-2-one.
Application
1-Acetylindole may be used in the stereocontrolled synthesis of (±)-geissoschizine. It may be used in the preparation of (1-acetyl-κO-indolyl-κC2)tetracarbonylmanganese, via a standard cyclomanganation procedure.
Reactant for preparation of:
Reactant for:
- Antimycobacterial agents
- Cyclin-dependent kinase (CDK2) inhibitors
Reactant for:
- C3-C3 oxidative cross-coupling reactions
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Regioselective acylations at the 2 and 6 position of N-acetylindole.
Cruz R, et al.
Tetrahedron Letters, 42(8), 1467-1469 (2001)
Mangenese (III) acetate oxidation of 1-acetylindole derivatives.
Izumi T, et al.
Journal of Heterocyclic Chemistry, 30(4), 1133-1136 (1993)
Vikas K Shukla et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 133, 626-638 (2014-07-06)
Quantum chemical calculations of ground state energy, geometrical structure and vibrational wavenumbers of 1-acetylindole were carried out using density functional (DFT/B3LYP) method with 6-311++G(d,p) basis set. The FT-IR and FT-Raman spectra were recorded in the condensed state. The fundamental vibrational
Synthesis and alkyne-coupling chemistry of cyclomanganated 1-and 3-acetylindoles, 3-formylindole and analogues.
Depree GJ, et al.
Journal of Organometallic Chemistry, 691(4), 667-679 (2006)
A concise, stereoselective synthesis of (?)-geissoschizine.
Bennasar M, et al.
Tetrahedron Letters, 37(50), 9105-9106 (1996)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service