All Photos(1)
About This Item
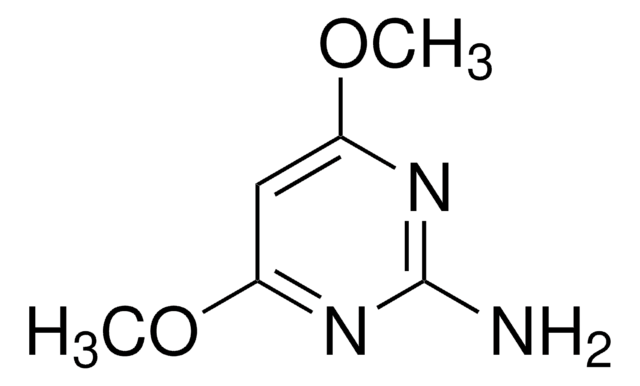
Empirical Formula (Hill Notation):
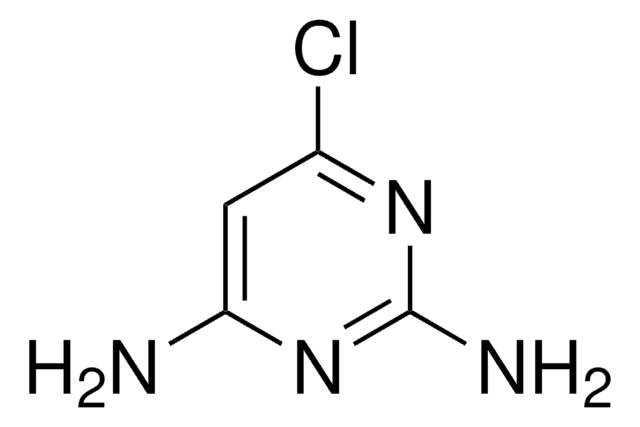
C6H9N3O2
CAS Number:
Molecular Weight:
155.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
149-152 °C (lit.)
solubility
methanol: soluble 25 mg/mL, clear, colorless to yellow
SMILES string
COc1cc(N)nc(OC)n1
InChI
1S/C6H9N3O2/c1-10-5-3-4(7)8-6(9-5)11-2/h3H,1-2H3,(H2,7,8,9)
InChI key
LNTJJKHTAZFVJJ-UHFFFAOYSA-N
Related Categories
General description
4-Amino-2,6-dimethoxypyrimidine is methoxy substituted 4-aminopyrimidine. Molecules of 4-amino-2,6-dimethoxypyrimidine are linked by an N-H.O hydrogen bond and an N-H.N hydrogen bond, forming sheets containing centrosymmetric rings. Photocatalytic degradation of 4-amino-2,6-dimethoxypyrimidine on TiO2 has been reported. Mass spectra of 4-amino-2,6-dimethoxypyrimidine has been studied.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Christopher Glidewell et al.
Acta crystallographica. Section C, Crystal structure communications, 59(Pt 4), O202-O204 (2003-04-12)
Molecules of the title compound, C(6)H(9)N(3)O(2), are linked by an N-H.O hydrogen bond [H.O = 2.29 A, N.O = 3.169 (2) A and N-H.O = 173 degrees ] and an N-H.N hydrogen bond [H.N = 2.12 A, N.N = 2.999
Photocatalytic transformations of aminopyrimidines on TiO< sub> 2</sub> in aqueous solution.
Calza P, et al.
Applied Catalysis. B, Environmental, 52(4), 267-274 (2004)
Mass spectra of methoxy-substituted 4-aminopyrimidines.
Khmel'nitskii RA, et al.
Chemistry of Heterocyclic Compounds, 10(1), 113-116 (1974)
Monica Olivella et al.
Archiv der Pharmazie, 348(1), 68-80 (2014-11-22)
New nitrosopyrimidines were synthesized and evaluated as potential antibacterial agents. Different compounds structurally related with 4,6-bis(alkyl or arylamino)-5-nitrosopyrimidines were evaluated. Some of these nitrosopyrimidines displayed significant antibacterial activity against human pathogenic bacteria. Among them compounds 1c, 2a-c, and 9a-c exhibited
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service