373761
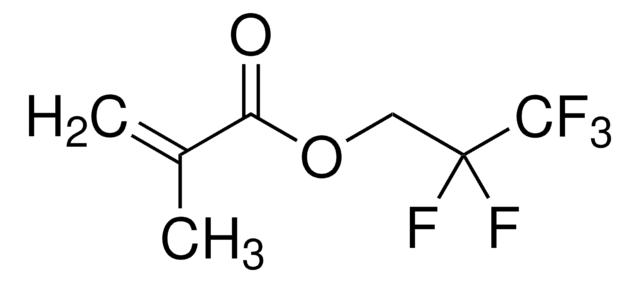
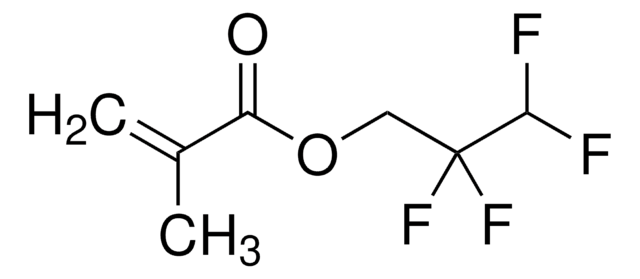
2,2,2-Trifluoroethyl methacrylate
contains 50-200 ppm MEHQ as inhibitor, 99%
Synonym(s):
2,2,2-Trifluoroethyl 2-methylprop-2-enoate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2C=C(CH3)CO2CH2CF3
CAS Number:
Molecular Weight:
168.11
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99%
form
liquid
contains
50-200 ppm MEHQ as inhibitor
refractive index
n20/D 1.361 (lit.)
bp
59 °C/100 mmHg (lit.)
density
1.181 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(=C)C(=O)OCC(F)(F)F
InChI
1S/C6H7F3O2/c1-4(2)5(10)11-3-6(7,8)9/h1,3H2,2H3
InChI key
QTKPMCIBUROOGY-UHFFFAOYSA-N
General description
2,2,2-Trifluoroethyl methacrylate (TFEMA) is a semifluorinated monomer that can be synthesized from methacryloyl chloride and 2,2,2-trifluoroethanol in presence of triethylamine. Its properties include chemical inertness, good wear resistance and low dielectric constant.
Application
TFEMA can be used in the preparation of poly(TFEMA), which can be used in the development of acrylic protective coatings.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Chronic 3 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
62.6 °F - closed cup
Flash Point(C)
17 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yoshinori Kadoma
Dental materials journal, 29(5), 602-608 (2010-09-10)
The kinetic polymerization behavior of 2,2,2-trifluoroethyl methacrylate (TFEMA), 1,1,1,3,3,3-hexafluoroisopropyl methacrylate (HFIPMA), 2,2,2-trifluoroethyl acrylate (TFEA) and 1,1,1,3,3,3-hexafluoroisopropyl acrylate (HFIPA) was determined by isothermal differential scanning calorimetry (DSC) and high-performance liquid chromatography (HPLC) in order to improve the properties of fluorinated powder-liquid
Arda Yurtsever et al.
Electrophoresis, 30(4), 589-598 (2009-01-22)
A new, fluorinated monolithic stationary phase for CEC was first synthesized by a single-stage, thermally initiated copolymerization of a fluorinated monomer, 2,2,2-trifluoroethyl methacrylate (TFEM) and ethylene dimethacrylate (EDMA) in the presence of a porogen mixture. In this preparation, 2-acrylamido-2-methyl-1-propanesulfonic acid
Grafting modification of ramie fibers with poly (2, 2, 2-trifluoroethyl methacrylate) via reversible addition-fragmentation chain transfer (RAFT) polymerization in supercritical carbon dioxide
Liu X, et al.
Reactive and Functional Polymers, 70(12), 972-979 (2010)
Preparation and surface properties of poly (2, 2, 2-trifluoroethyl methacrylate) coatings modified with methyl acrylate
Xu A, et al.
Journal of Coatings Technology and Research, 13(5), 795-804 (2016)
Yoshinori Kadoma et al.
Dental materials journal, 28(5), 642-648 (2009-10-14)
In order to clarify the effect of fluorination of an adhesive resin on the durability of the resin bond to precious metal alloys, 2,2,2-trifluoroethyl methacrylate (TFEMA)-poly(2,2,2-trifluoroethyl methacrylate) (PTFEMA)/TBBO adhesive resin was prepared. The tensile bond strength of this resin to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service