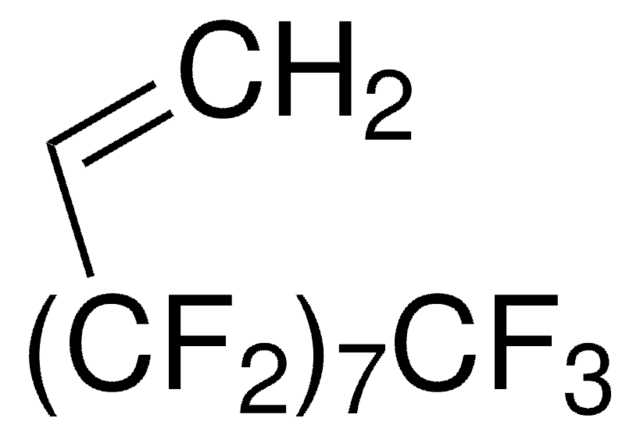371459
1H,1H,2H-Perfluoro-1-hexene
99%
Synonym(s):
3,3,4,4,5,5,6,6,6-Nonafluoro-1-hexene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH2=CH(CF2)3CF3
CAS Number:
Molecular Weight:
246.07
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
8.5 (vs air)
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.3 (lit.)
bp
59-60 °C (lit.)
density
1.452 g/mL at 25 °C (lit.)
SMILES string
FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C=C
InChI
1S/C6H3F9/c1-2-3(7,8)4(9,10)5(11,12)6(13,14)15/h2H,1H2
InChI key
GVEUEBXMTMZVSD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T Nakayama et al.
The journal of physical chemistry. A, 111(5), 909-915 (2007-02-03)
FTIR-smog chamber techniques were used to study the products of the Cl atom and OH radical initiated oxidation of CF3CH=CH2 in 700 Torr of N2/O2, diluent at 296 K. The Cl atom initiated oxidation of CF3CH=CH2 in 700 Torr of
Linda Angevine Malley et al.
Inhalation toxicology, 14(8), 773-787 (2002-07-18)
Inhalation studies were conducted to determine the potential subchronic toxicity of a mixture of trans-1,2-dichloroethylene (70%), cis-1,2-dichloroethylene (5%), and perfluorobutylethylene (25%). Groups of rats were exposed to 0, 400, 2000, or 8000 ppm concentrations of the mixture vapor 6 h/day
G L Kennedy
Critical reviews in toxicology, 21(2), 149-170 (1990-01-01)
Fluorine-containing monomers form the basis for production of a large number of commercially important polymers. Most of the polymerization occurs as gas-phase reactions, hence the hazards associated with the monomers arises primarily from inhalation. The chemicals covered in this review
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service