368113
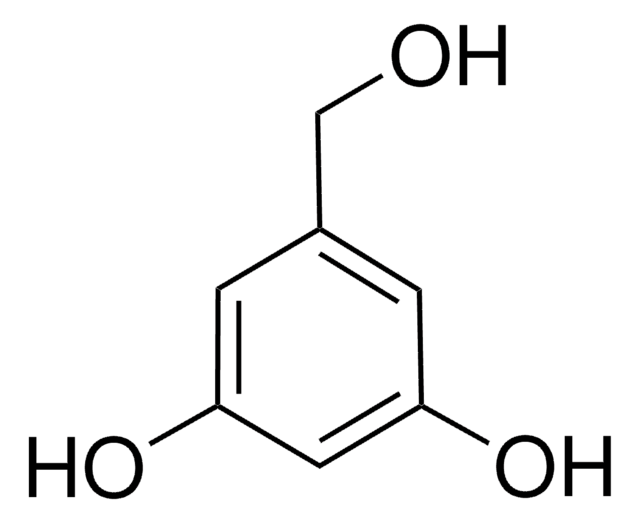
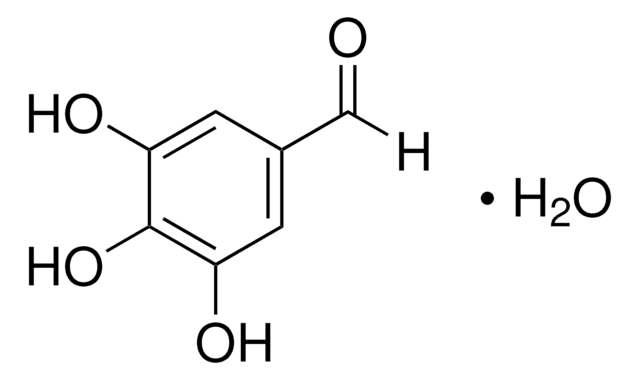
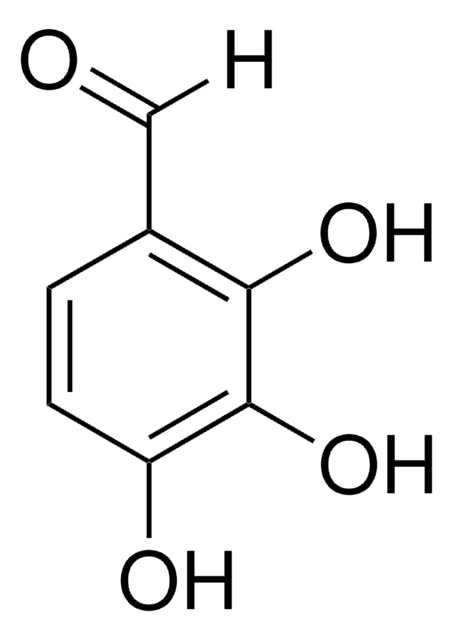
3,5-Dihydroxybenzaldehyde
98%
Synonym(s):
α-Resorcylaldehyde (6CI)
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(HO)2C6H3CHO
CAS Number:
Molecular Weight:
138.12
Beilstein:
1930147
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
153-158 °C (lit.)
SMILES string
[H]C(=O)c1cc(O)cc(O)c1
InChI
1S/C7H6O3/c8-4-5-1-6(9)3-7(10)2-5/h1-4,9-10H
InChI key
HAQLHRYUDBKTJG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Condensation of isopropyl ethers of p-hydroxyphenyl acetic acid and 3,5-dihydroxybenzaldehyde has been reported.
Application
3,5-Dihydroxybenzaldehyde is suitable for use in the synthesis of 3,5-dihydroxyphenylglycine (3,5-DHPG). It may be used in the synthesis of:
- fulgide7
- 3,5-bis(undeca-4,6-diynyloxy)benzaldehyde
- 3,5-didodecyloxybenzaldehyde
- 1′-methyl-1′,5′-dihydro-2′-(3,5-bis(undeca-4,6-diynyloxy)phenyl)-1H-pyrrolo[3′,4′:1,9](C60-Ih)[5,6]fullerene (F2D)
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A re-investigation of resveratrol synthesis by Perkins reaction. Application to the synthesis of aryl cinnamic acids.
Solladie G, et al.
Tetrahedron, 59(18), 3315-3321 (2003)
A Porel et al.
Indian journal of pharmaceutical sciences, 73(1), 46-56 (2011-12-02)
The aim of the present study was the development and subsequent validation of a simple, precise and stability-indicating reversed phase HPLC method for the simultaneous determination of guaifenesin, terbutaline sulphate and bromhexine hydrochloride in the presence of their potential impurities
Denis E Markov et al.
The journal of physical chemistry. A, 109(24), 5266-5274 (2006-07-15)
Exciton diffusion and photoluminescence quenching in conjugated polymer/fullerene heterostructures are studied by time-resolved photoluminescence. It is observed that heterostructures consisting of a spin-coated poly(p-phenylene vinylene) (PPV)-based derivative and evaporated C60 are ill-defined because of diffusion of C60 into the polymer
Enzymatic resolution and pharmacological activity of the enantiomers of 3, 5-dihydroxyphenylglycine, a metabotropic glutamate receptor agonist.
Richard BS, et al.
Bioorganic & Medicinal Chemistry Letters, 5(3), 223-228 (1995)
Synthesis and photochromism of E, E-3, 4-(3, 5-dimethoxybenzylidene) succinic anhydride and its infra red active 2-dicyanomethylene derivative.
Asiri AM.
Journal of Photochemistry and Photobiology A: Chemistry, 159(1), 1-5 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service