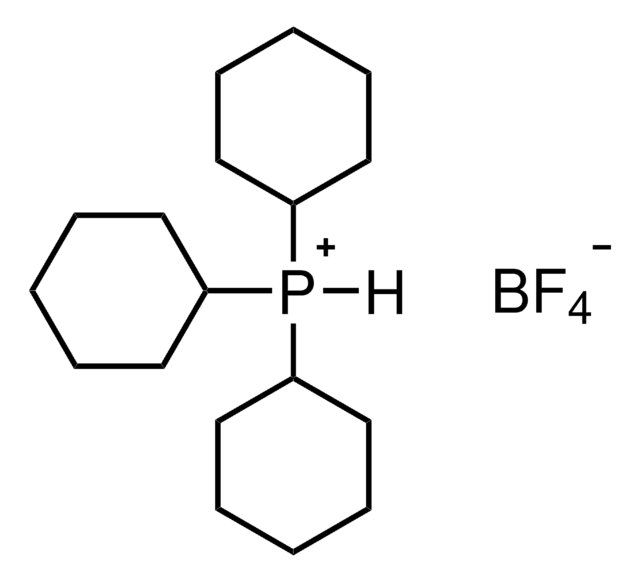
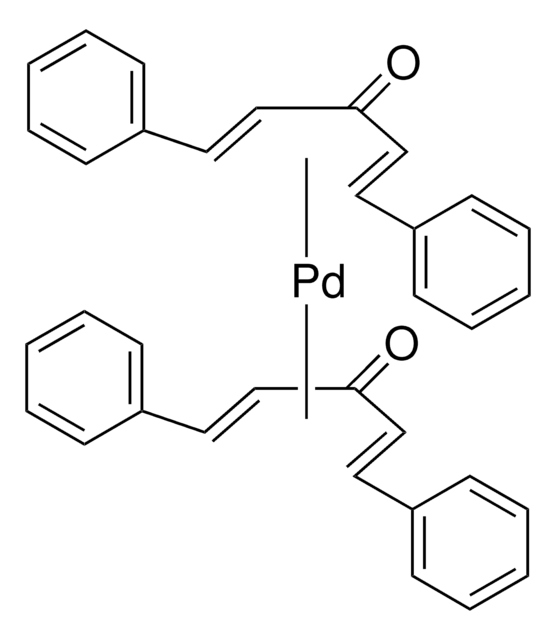
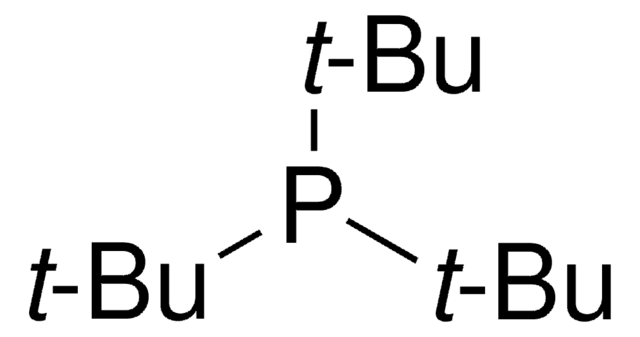366315
Tris(dibenzylideneacetone)dipalladium(0)-chloroform adduct
Synonym(s):
Dipalladium-tris(dibenzylideneacetone)chloroform complex, Pd2(dba)3 · CHCl3
About This Item
Recommended Products
form
solid
Quality Level
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
131-135 °C (lit.)
SMILES string
[Pd].[Pd].ClC(Cl)Cl.O=C(/C=C/c1ccccc1)\C=C\c2ccccc2.O=C(/C=C/c3ccccc3)\C=C\c4ccccc4.O=C(\C=C\c5ccccc5)/C=C/c6ccccc6
InChI
1S/3C17H14O.CHCl3.2Pd/c3*18-17(13-11-15-7-3-1-4-8-15)14-12-16-9-5-2-6-10-16;2-1(3)4;;/h3*1-14H;1H;;/b3*13-11+,14-12+;;;
InChI key
LNAMMBFJMYMQTO-FNEBRGMMSA-N
General description
Application
- To compose the catalytic system for the preparation of homoallylpalladium complexes.These complexes underwent in situ Stille type cross coupling with various vinyltin reagents to afford the cyclized products bearing allyl appendages.
- As palladium source in the asymmetric transformations of 3,4-epoxy-1-butene.
- As catalyst for the Heck cross-coupling reaction of iodobenzene with styrene.
- As cyclization catalyst.
- As catalyst for [2+2+2] cycloaddtion of didehydrotriphenylenes to the corresponding extended triphenylenes.
- As catalyst for the carbonylation of b,b-imidoyl iodides to the corresponding imidate esters used, in turn, to prepare cyclic, quaternary amino acids.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
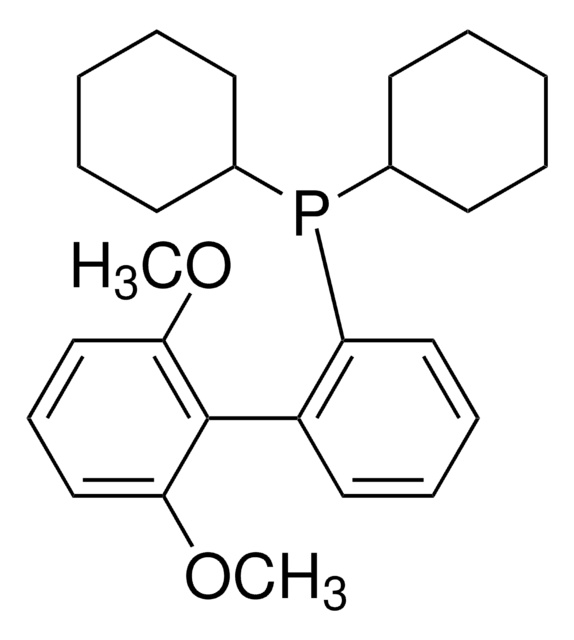
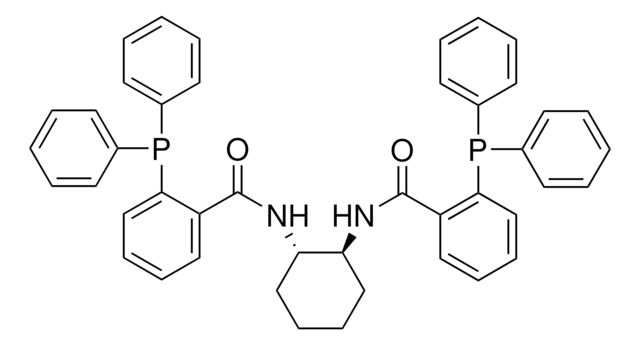
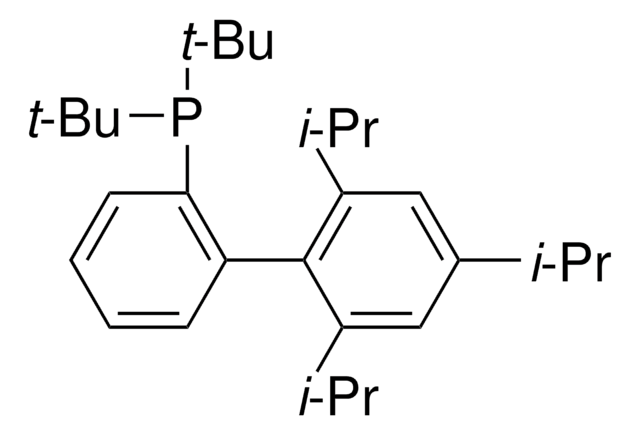
Customers Also Viewed
Articles
A variety of transition-metal catalysts for the Suzuki coupling reaction are now available in our catalog. The majority of these catalysts are palladium- and nickelbased, typically utilizing phosphine-derived ligands.
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)