All Photos(1)
About This Item
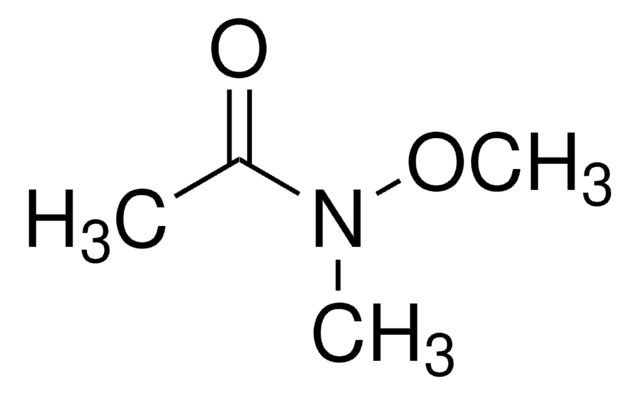
Linear Formula:
ClCH2CON(OCH3)CH3
CAS Number:
Molecular Weight:
137.56
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
39-41 °C (lit.)
storage temp.
2-8°C
SMILES string
CON(C)C(=O)CCl
InChI
1S/C4H8ClNO2/c1-6(8-2)4(7)3-5/h3H2,1-2H3
InChI key
SCOJKGRNQDKFRP-UHFFFAOYSA-N
Related Categories
General description
2-Chloro-N-methoxy-N-methylacetamide is a Weinreb amide.
Application
2-Chloro-N-methoxy-N-methylacetamide may be used in the preparation of:
- 2-heptyl-3-hydroxy-4(1H)-quinolone (Pseudomonas quinolone signal or PQS) and structurally related 2-alkyl-4-quinolones having biological activity
- 2-(benzo[d]thiazol-2-ylsulfonyl)-N-methoxy-N-methylacetamide
- α-chloro-ketone, starting reagent for the one-pot synthesis of 2-heptyl-3-hydroxyl-4(1H)-quinolone (PQS), signalling molecule in the quorum sensing of Pseudomonas aeruginosa
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
219.2 °F - closed cup
Flash Point(C)
104 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
James T Hodgkinson et al.
Nature protocols, 7(6), 1184-1192 (2012-05-29)
An optimized procedure for the efficient preparation of 2-heptyl-3-hydroxy-4(1H)-quinolone (Pseudomonas quinolone signal or PQS) and a diverse range of structurally related 2-alkyl-4-quinolones with biological activity is presented. The two-step synthesis begins with the formation of α-chloro ketones by the coupling
James T Hodgkinson et al.
Organic & biomolecular chemistry, 9(1), 57-61 (2010-10-23)
Expedient syntheses of Pseudomonas quinolone signal (PQS) and related structural analogues using microwave and flow methods are reported.
New Reagent for Convenient Access to the a, ?-Unsaturated N-Methoxy-N-methyl-amide Functionality by a Synthesis Based on the Julia Olefination Protocol.
Narayana Manjunath B, et al
European Journal of Organic Chemistry, 12, 2851-2855 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service