360716
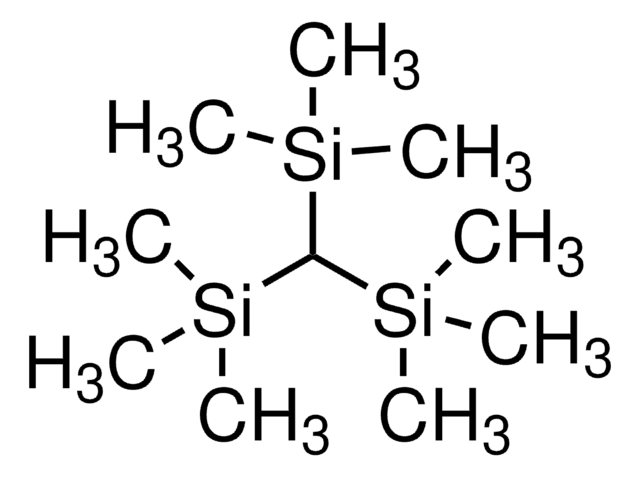
Tris(trimethylsilyl)silane
97%
Synonym(s):
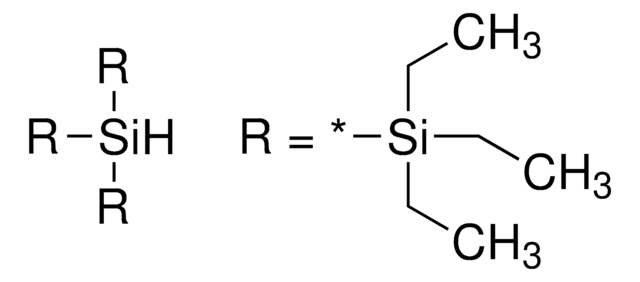
1,1,1,3,3,3-Hexamethyl-2-trimethylsilyl-trisilane, TTMSS
About This Item
Recommended Products
Quality Level
Assay
97%
form
liquid
reaction suitability
reagent type: reductant
refractive index
n20/D 1.489 (lit.)
bp
73 °C/5 mmHg (lit.)
density
0.806 g/mL at 25 °C (lit.)
SMILES string
C[Si](C)(C)[SiH]([Si](C)(C)C)[Si](C)(C)C
InChI
1S/C9H28Si4/c1-11(2,3)10(12(4,5)6)13(7,8)9/h10H,1-9H3
InChI key
SQMFULTZZQBFBM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Used in:
- Hydrosilylations
- Radical reactions
- Reductions of acid chlorides
- Reductions of carbon-halogen bonds
- Hydrosilations of carbonyls
- Another common application involves the use of the tris(trimethylsilyl)silyl (TTMSS, or super silyl) group when complexed with transition metals and main group elements
- More recently, the super silyl group is being utilized in carbon–carbon bond forming reactions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
131.0 °F - closed cup
Flash Point(C)
55 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The super silyl group is a powerful tool for the synthetic chemist, showing great efficacy in various carbon–carbon bond forming reactions.
This article briefly reviews the methods and mechanisms for the formation of molecular monolayers on silicon surfaces, the properties of these monolayers and current perspectives regarding their application in molecular electronic and sensing applications.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service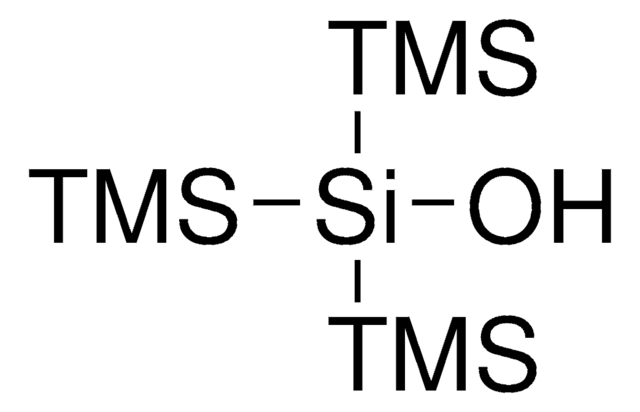

![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)

![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)