All Photos(1)
About This Item
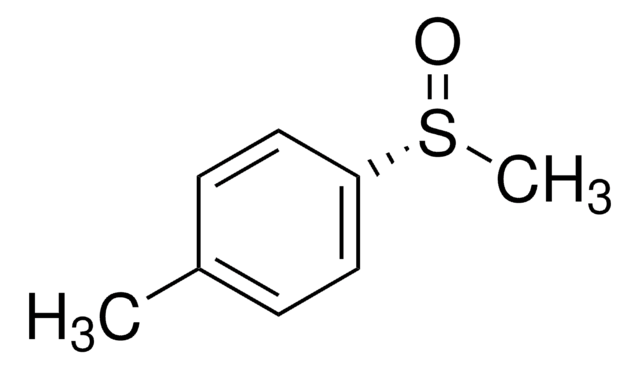
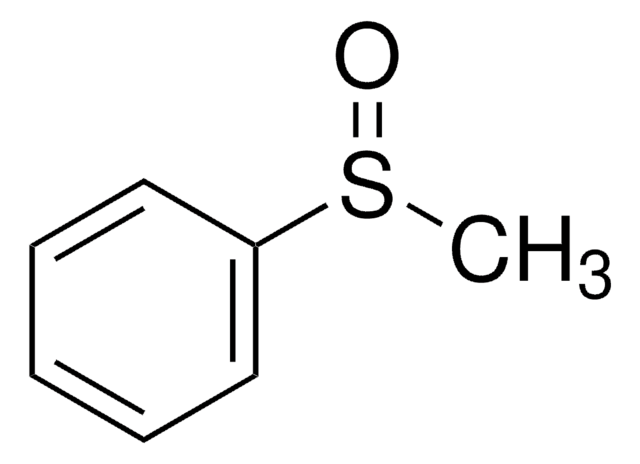
Linear Formula:
CH3C6H4S(O)CH3
CAS Number:
Molecular Weight:
154.23
Beilstein:
2324696
MDL number:
UNSPSC Code:
12191600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
optical activity
[α]20/D −145°, c = 2 in acetone
optical purity
ee: 99% (HPLC)
mp
75-77 °C (lit.)
SMILES string
Cc1ccc(cc1)S(C)=O
InChI
1S/C8H10OS/c1-7-3-5-8(6-4-7)10(2)9/h3-6H,1-2H3/t10-/m0/s1
InChI key
FEVALTJSQBFLEU-JTQLQIEISA-N
Looking for similar products? Visit Product Comparison Guide
Application
(S)-(-)-Methyl p-tolyl sulfoxide can be used as a nucleophilic reagent to synthesize:
- Optically active β-disulfoxides by reacting with arenesulfinic esters via formation of α-sulfinylcarbanion.
- α-substituted N-hydroxylamines by treating with nitrones via preparation of (S)-(-)-methyl p-tolyl sulfoxide anion.
- 2-O-benzyl-3,4-O-isopropylidene-L-erythrose by one-carbon homologation of 2,3-O-isopropylidene-L-glyceraldehyde.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
β -Disulfoxides. II. The Preparation of Some Optically Active β -Disulfoxides
Kunieda N, et al.
Bulletin of the Chemical Society of Japan, 49(1), 256-259 (1976)
A highly stereoselective synthesis of d-erythrose derivatives by one-carbon homologation of 2, 3-O-isopropylidene-d-glyceraldehyde with (R)-methyl p-tolyl sulfoxide.
Arroyo-Gomez Y, et al.
Tetrahedron Asymmetry, 11(3), 789-796 (2000)
The reaction of nitrones with (R)-(+)-methyl p-tolyl sulfoxide anion; asymmetric synthesis of optically active secondary amines.
Murahashi S-I, et al.
Tetrahedron Letters, 34(16), 2645-2648 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service