335975
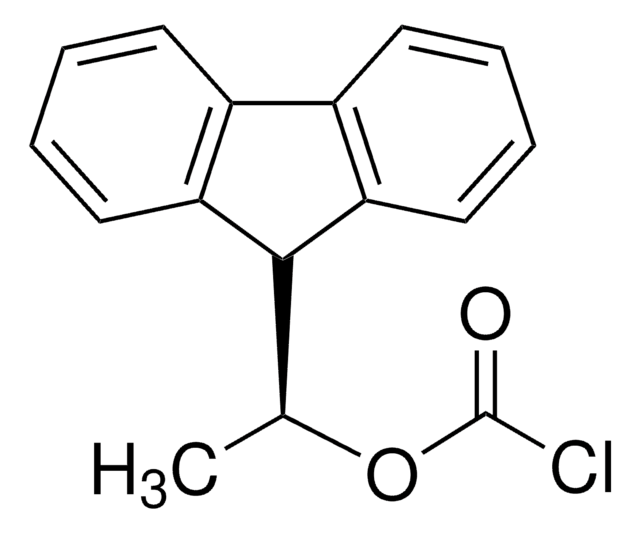
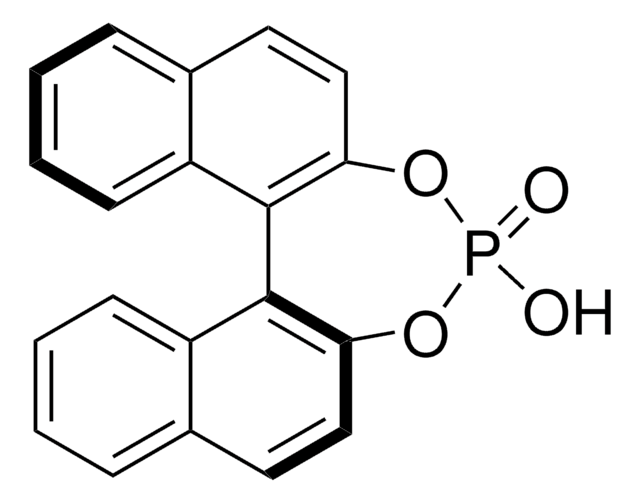
(+)-1-(9-Fluorenyl)ethyl chloroformate solution
18 mM in acetone, for chiral derivatization
Synonym(s):
(+)-FLEC solution
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H13ClO2
CAS Number:
Molecular Weight:
272.73
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
for chiral derivatization
Quality Level
vapor density
2 (vs air)
vapor pressure
180 mmHg ( 20 °C)
form
liquid
concentration
18 mM in acetone
refractive index
n20/D 1.3602
density
0.79 g/mL at 25 °C
storage temp.
2-8°C
InChI
1S/C16H13ClO2/c1-10(19-16(17)18)15-13-8-4-2-6-11(13)12-7-3-5-9-14(12)15/h2-10,15H,1H3
InChI key
SFRVOKMRHPQYGE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
(+)-1-(9-Fluorenyl)ethyl chloroformate is a highly fluorescent compound1 commonly used as a chiral derivatizing agent for the separation of racemates prior to reversed-phase HPLC analysis.
Application
- Chiral analysis of β-methylamino alanine (BMAA) enantiomers: Details the use of (+)-1-(9-fluorenyl)-ethyl chloroformate (FLEC) for derivatization followed by LC-MS/MS analysis, improving the understanding of amino acids′ stereochemistry (Zurita et al., 2019).
- Enantioselective micellar electrokinetic chromatography of dl‐amino acids: Utilizes (+)-1-(9-fluorenyl)ethyl chloroformate derivatization combined with UV-induced fluorescence detection to analyze amino acids, enhancing analytical methodologies (Prior et al., 2018).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - STOT SE 3
Target Organs
Central nervous system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
1.4 °F
Flash Point(C)
-17 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Reversed-phase high-performance liquid chromatographic analysis of atenolol enantiomers in plasma after chiral derivatization with (+)-1-(9-fluorenyl) ethyl chloroformate.
Rosseel MT, et al.
Journal of Chromatography. B, Biomedical Applications, 568(1), 239-245 (1991)
E Frigerio et al.
Journal of chromatography. A, 660(1-2), 351-358 (1994-02-04)
A sensitive and selective high-performance liquid chromatographic method for the determination of reboxetine enantiomers in human plasma was developed. Although two chiral centres are present in reboxetine, its stereospecific synthesis leads to two rather than four possible enantiomers. After extraction
Y Bergqvist et al.
Journal of chromatography, 652(1), 73-81 (1994-01-14)
A sensitive, stereoselective and rapid reversed-phase liquid chromatographic method for the determination of (SR)- and (RS)-mefloquine enantiomers in 100 microliters plasma and capillary blood collected on chromatographic paper is presented. The assay involves protein precipitation from plasma, liquid-liquid extraction of
Radu-Cristian Moldovan et al.
Journal of chromatography. A, 1590, 80-87 (2019-01-15)
D-amino acids (AA) analysis is becoming more and more relevant for metabolomics, therefore new analytical tools need to be developed. A common approach to achieve AA enantioseparation is chiral derivatization. Among the chiral derivatization reagents, (+) or (-)-1-(9-fluorenyl) ethyl chloroformate
Radu-Cristian Moldovan et al.
Journal of chromatography. A, 1467, 400-408 (2016-08-25)
In the context of bioanalytical method development, process automatization is nowadays a necessity in order to save time, improve method reliability and reduce costs. For the first time, a fully automatized micellar electrokinetic chromatography-mass spectrometry (MEKC-MS) method with in-capillary derivatization
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service