327956
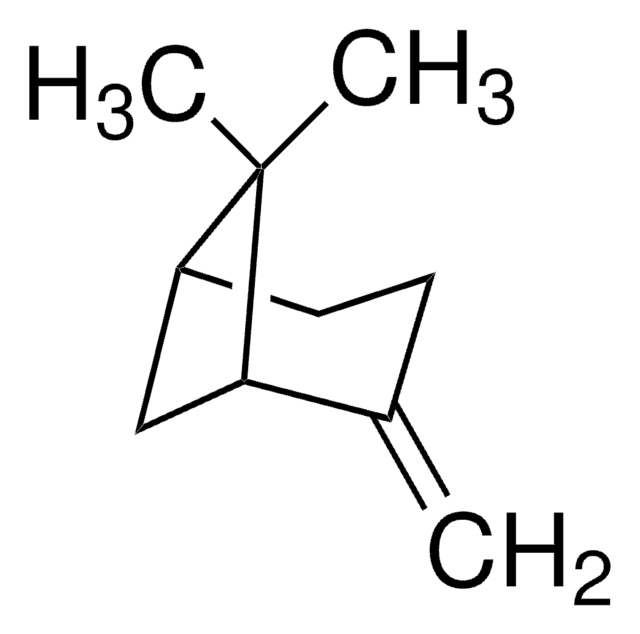
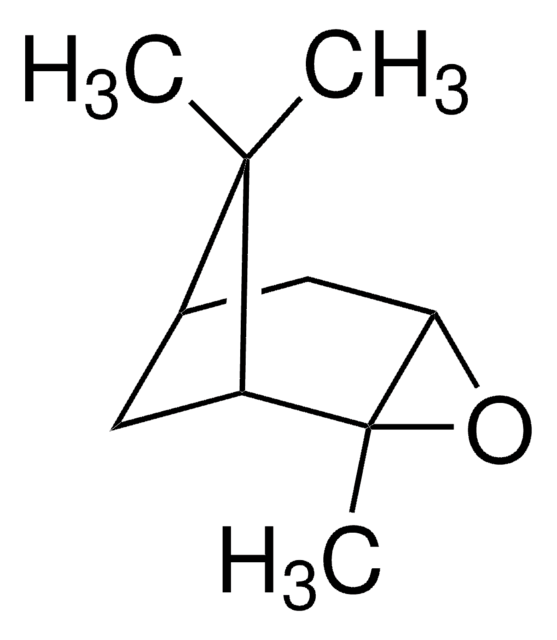
(1R)-(+)-Nopinone
98%
Synonym(s):
6,6-Dimethylbicyclo[3.1.1]heptan-2-one
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H14O
CAS Number:
Molecular Weight:
138.21
MDL number:
UNSPSC Code:
12352115
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
optical activity
[α]20/D +16°, neat
refractive index
n20/D 1.479 (lit.)
bp
209 °C (lit.)
density
0.981 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC1(C)[C@H]2CCC(=O)[C@@H]1C2
InChI
1S/C9H14O/c1-9(2)6-3-4-8(10)7(9)5-6/h6-7H,3-5H2,1-2H3/t6-,7-/m0/s1
InChI key
XZFDKWMYCUEKSS-BQBZGAKWSA-N
Application
(1R)-(+)-Nopinone may be used in the preparation of a chiral annulated indene derivative, which can be a potentially useful chiral ligand for transition metal complexes in asymmetric transformations. It may also react with secondary amines in cyclohexane to form the corresponding enamines.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
167.0 °F - closed cup
Flash Point(C)
75 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Boranes in synthesis-VII. Synthesis of 2-dialkylamino-6, 6-dimethylbicyclo [3.1. 1] heptan-3-ols from (R)-(+)-nopinone. Chiral auxiliaries for the addition of diethylzinc to aromatic aldehydes.
Goralski CT, et al.
Tetrahedron Asymmetry, 8(23), 3863-3871 (1997)
Synthesis of (1R)-(+)-nopinone-and (1S)-(-)-verbenone-derived chiral annulated indenes via electrocyclic reactions.
Liu C and Sowa JR.
Tetrahedron Letters, 37(40), 7241-7244 (1996)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service