326755
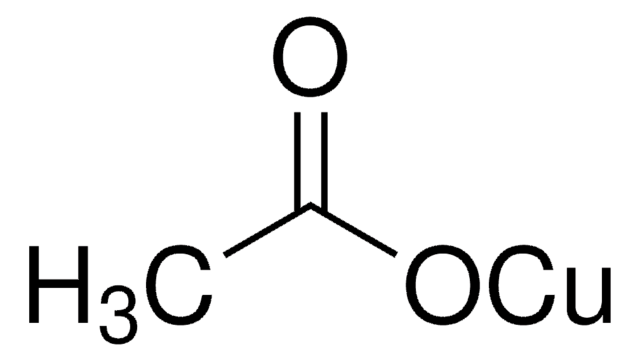
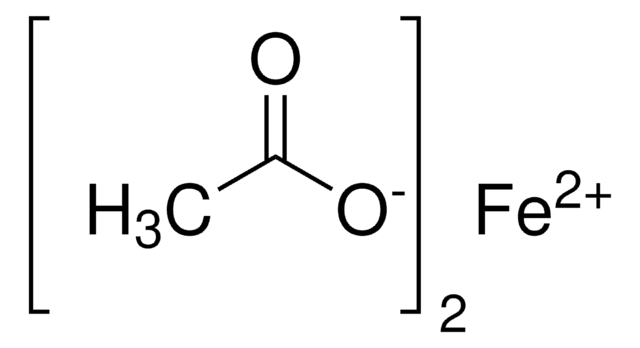
Copper(II) acetate
98%
Synonym(s):
Cupric acetate
About This Item
Recommended Products
vapor density
6.9 (vs air)
Quality Level
Assay
98%
form
powder or crystals
reaction suitability
reaction type: click chemistry
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
greener alternative category
, Aligned
SMILES string
CC(=O)O[Cu]OC(C)=O
InChI
1S/2C2H4O2.Cu/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
InChI key
OPQARKPSCNTWTJ-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Copper(II) acetate also known as cupric acetate, can be used as a catalyst in various processes in the field of greener chemistry. It is particularly useful in cross-coupling reactions, where it can promote the formation of carbon-carbon or carbon-heteroatom bonds, without the need for hazardous reagents or solvents
Application
Copper-catalyzed reductive amination of aromatic and aliphatic ketones with anilines using environmental-friendly molecular hydrogen
Copper(II) acetate is used as a catalyst:
- In the N-arylation of α-amino esters with p-tolylboronic acid to synthesize biaryls via cross-coupling reactions
- In the the synthesis of substituted isoxazole derivatives
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
does not flash
Flash Point(C)
does not flash
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service