All Photos(1)
About This Item
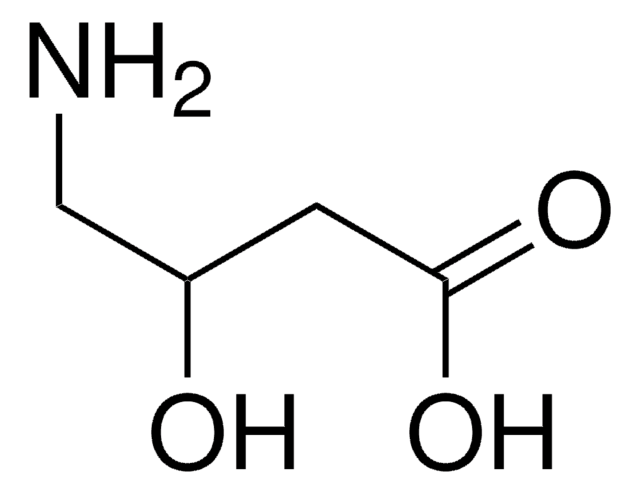
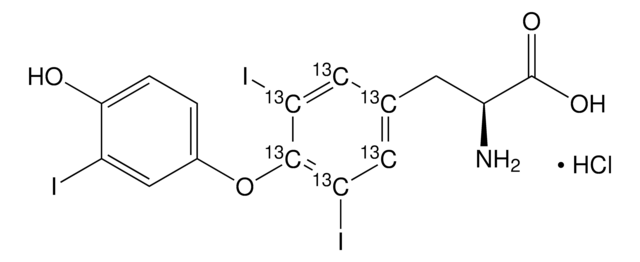
Linear Formula:
CF3CH(NH2)CO2H
CAS Number:
Molecular Weight:
143.06
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
reaction suitability
reaction type: solution phase peptide synthesis
mp
231-234 °C (lit.)
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
NC(C(O)=O)C(F)(F)F
InChI
1S/C3H4F3NO2/c4-3(5,6)1(7)2(8)9/h1H,7H2,(H,8,9)
InChI key
HMJQKIDUCWWIBW-UHFFFAOYSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
L Pollegioni et al.
FEBS letters, 507(3), 323-326 (2001-11-07)
D-Amino acid oxidase (DAAO) is a flavoprotein oxidase that catalyzes the oxidation of amino acids and produces ketoacids and H(2)O(2). The rate of product release from reduced DAAO from Rhodotorula gracilis is pH dependent and reflects a pK(a) of approximately
C W Fearon et al.
Biochemistry, 21(16), 3790-3794 (1982-08-03)
Inactivation of gamma-cystathionase by beta, beta, beta-trifluoroalanine, a suicide inactivator of the enzyme, results in covalent labeling of an amino group of the protein [Silverman, R. B., & Abeles, R. H. (1977) Biochemistry 16, 5515-5520]. We have established that this
R S Phillips et al.
Archives of biochemistry and biophysics, 296(2), 489-496 (1992-08-01)
Trifluoroalanine is a mechanism-based inactivator of Escherichia coli tryptophan indole-lyase (tryptophanase) and E. coli tryptophan synthase (R. B. Silverman and R. H. Abeles, 1976, Biochemistry 15, 4718-4723). We have found that indole is able to prevent inactivation of tryptophan indole-lyase
E A Wang et al.
Biochemistry, 20(26), 7539-7546 (1981-12-22)
The alanine racemase from Escherichia coli B has been shown to process DL isomers of beta -fluoroalanine as suicide substrates with an identical partitioning ratio for each enantiomer of 820 catalytic eliminations of HF per enzymatic inactivation event [Wang, E.
W S Faraci et al.
Biochemistry, 28(2), 431-437 (1989-01-24)
The alanine racemases are a group of PLP-dependent bacterial enzymes that catalyze the racemization of alanine, providing D-alanine for cell wall synthesis. Inactivation of the alanine racemases from the Gram-negative organism Salmonella typhimurium and Gram-positive organism Bacillus stearothermophilus with beta
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service