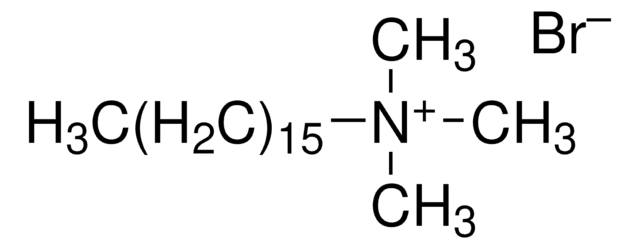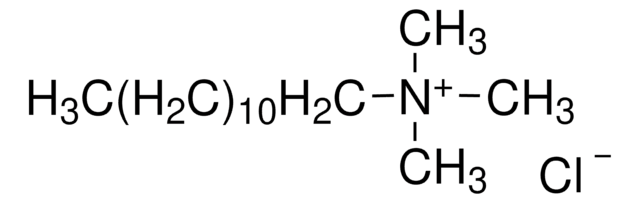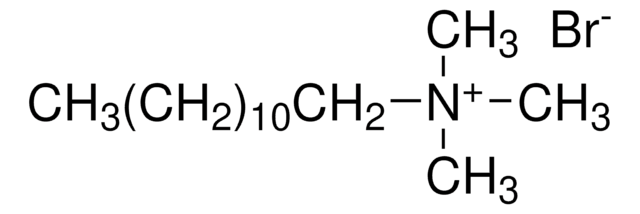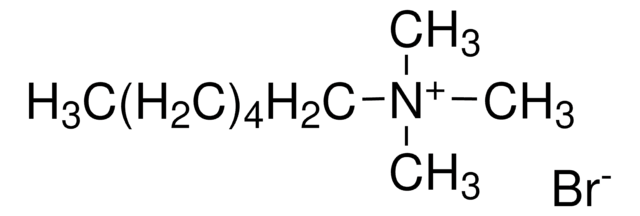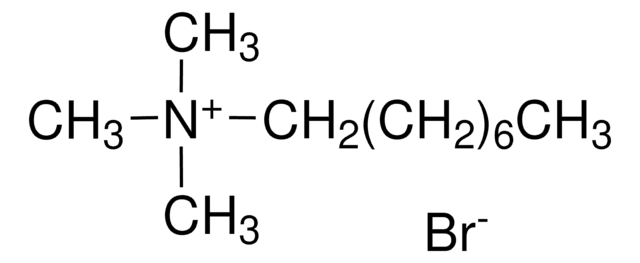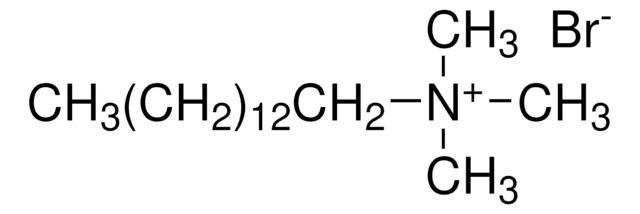30725
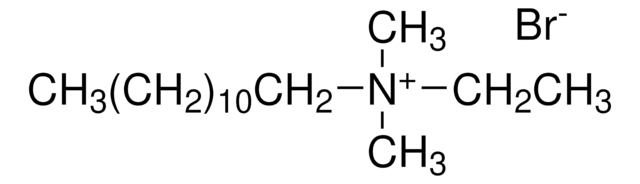
Decyltrimethylammonium bromide
≥98.0% (NT)
Synonym(s):
N,N,N-Trimethyl-1-decanaminium bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)9N(CH3)3(Br)
CAS Number:
Molecular Weight:
280.29
Beilstein:
3915222
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (NT)
SMILES string
[Br-].CCCCCCCCCC[N+](C)(C)C
InChI
1S/C13H30N.BrH/c1-5-6-7-8-9-10-11-12-13-14(2,3)4;/h5-13H2,1-4H3;1H/q+1;/p-1
InChI key
PLMFYJJFUUUCRZ-UHFFFAOYSA-M
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Miguel Jorge
Langmuir : the ACS journal of surfaces and colloids, 24(11), 5714-5725 (2008-05-06)
In this paper, a molecular dynamics simulation of surfactant self-assembly using realistic atomistic models is presented. The simulations are long enough to enable the observation of several processes leading to equilibrium, such as monomer addition and detachment, micelle dissolution, and
Zsombor Feldötö et al.
Langmuir : the ACS journal of surfaces and colloids, 24(7), 3348-3357 (2008-02-13)
The interaction between mucin and ions has been investigated by employing the quartz crystal microbalance technique with measurement of energy dissipation. The study was partially aimed at understanding the adsorption of mucin on surfaces with different chemistry, and for this
Nina M Kovalchuk et al.
Langmuir : the ACS journal of surfaces and colloids, 35(28), 9184-9193 (2019-07-04)
The coalescence of two different drops, one surfactant-laden and the other surfactant-free, was studied under the condition of confined flow in a microchannel. The coalescence was accompanied by penetration of the surfactant-free drop into the surfactant-laden drop because of the
Qian Zhao et al.
Environmental science & technology, 46(7), 3999-4007 (2012-03-01)
Organoclays synthesized from single chain quaternary ammonium cations (QAC) ((CH(3))(3)NR(+)) exhibit different mechanisms for the sorption of nonpolar organic compounds as the length of the carbon chain is increased. The interaction between a nonpolar sorbate and an organoclay intercalated with
Jonas Carlstedt et al.
Langmuir : the ACS journal of surfaces and colloids, 28(5), 2387-2394 (2012-01-06)
Full equilibrium phase diagrams are presented for two ternary systems composed of the cationic surfactant dodecyltrimethylammonium bromide (DTAB), water (D(2)O), and a cyclodextrin, either β-cyclodextrin (β-CD) or (2-hydroypropyl)-β-cyclodextrin (2HPβCD). (2)H NMR, SAXS, WAXS, and visual examination were used to determine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service