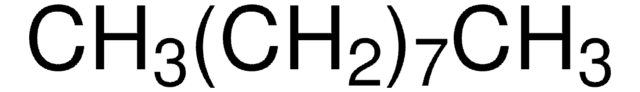All Photos(1)
About This Item
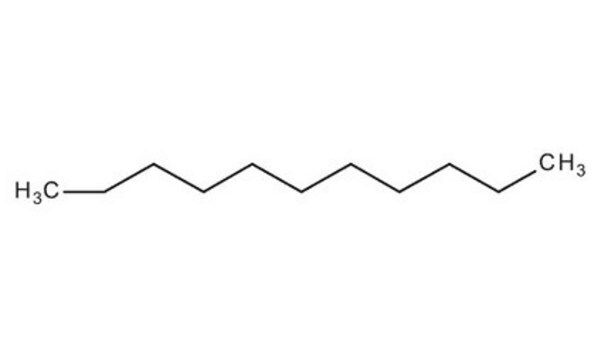
Linear Formula:
CH3(CH2)8CH3
CAS Number:
Molecular Weight:
142.28
Beilstein:
1696981
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
4.9 (vs air)
Quality Level
vapor pressure
1 mmHg ( 16.5 °C)
3.77 mmHg ( 37.7 °C)
Assay
≥95%
form
liquid
autoignition temp.
410 °F
expl. lim.
2.6 %
refractive index
n20/D 1.411 (lit.)
n20/D 1.412
bp
170.0-182.0 °C
174 °C (lit.)
mp
−30 °C (lit.)
density
0.730 g/mL at 20 °C
0.73 g/mL at 25 °C (lit.)
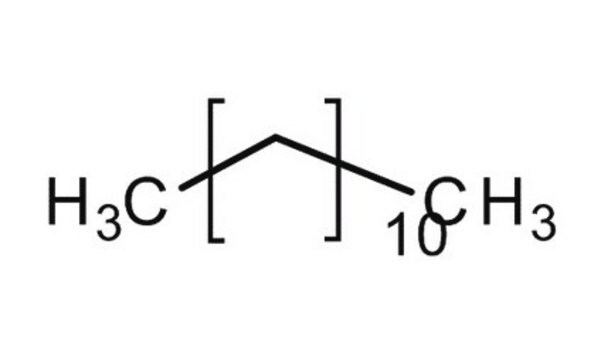
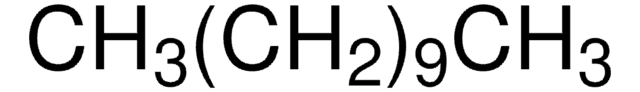
SMILES string
CCCCCCCCCC
InChI
1S/C10H22/c1-3-5-7-9-10-8-6-4-2/h3-10H2,1-2H3
InChI key
DIOQZVSQGTUSAI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Decane is an organic solvent. Bifunctional conversion of decane over Pt/ZSM-22, Pt/ZSM-5 and Pt/USY catalysts was compared. Structure of single-phase (sodium di-2-ethylsulfosuccinate)/D2O/decane microemulsions was investigated by small-angle neutron scattering.
Application
Decane was used as solvent to investigate the self-association of cyclohexanols.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 3
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
114.8 °F - closed cup
Flash Point(C)
46.0 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Self-association of cyclohexanols in inert solvents. Apparent heat capacities of cyclohexanol and substituted cyclohexanols in n-heptane and n-decane.
Trejo LM, et al.
J. Chem. Soc., Faraday, 87(11), 1739-1743 (1991)
Structure of Dense Sodium Di-2-Ethylsulfosuccinate/D 2 O/Decane Microemulsions.
Kotlarchyk M, et al.
Physical Review Letters, 53(9), 941-941 (1984)
Selective conversion of decane into branched isomers: A comparison of platinum/ZSM-22, platinum/ZSM-5 and platinum/USY zeolite catalysts.
Martens JA, et al.
Applied Catalysis, 76(1), 95-116 (1991)
Mahvash Karimi et al.
Colloids and surfaces. B, Biointerfaces, 95, 129-136 (2012-03-27)
Wettability alteration is considered to be one of the important mechanisms that lead to increased oil recovery during microbial enhanced oil recovery (MEOR) processes. Changes in wettability will greatly influence the petrophysical properties of the reservoir rocks and determine the
Ching-Wei Njauw et al.
Langmuir : the ACS journal of surfaces and colloids, 29(12), 3879-3888 (2013-02-28)
It has been known that the addition of bile salts to lecithin organosols induces the formation of reverse wormlike micelles and that the worms are similar to long polymer chains that entangle each other to form viscoelastic solutions. In this
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service