300810
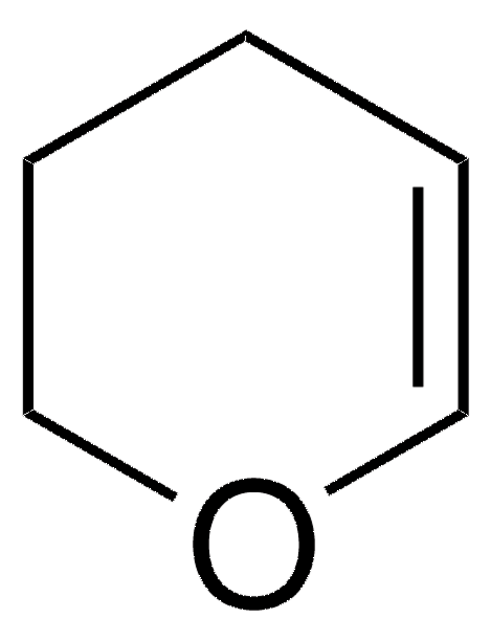
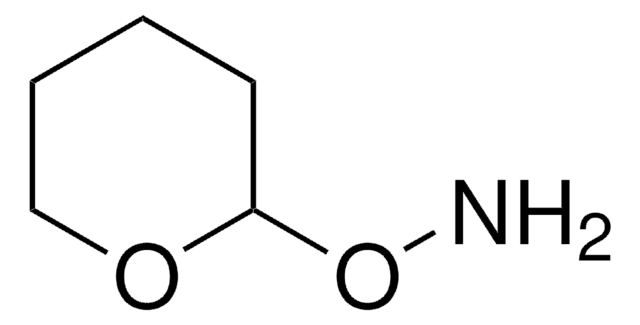
Tetrahydro-2-(2-propynyloxy)-2H-pyran
98%
Synonym(s):
(2-Propynyloxy)tetrahydropyran, (±)-Tetrahydro-2-(2-propynyloxy)-2H-pyran, 1-(2′-Tetrahydropyranyloxy)-2-propyne, 1-(Tetrahydro-2H-pyranyloxy)-2-propyne, 1-(Tetrahydropyranyloxy)prop-2-yne, Oct-2-yne-1-ol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H12O2
CAS Number:
Molecular Weight:
140.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.458 (lit.)
bp
63-65 °C/9 mmHg (lit.)
density
0.997 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C#CCOC1CCCCO1
InChI
1S/C8H12O2/c1-2-6-9-8-5-3-4-7-10-8/h1,8H,3-7H2
InChI key
HQAXHIGPGBPPFU-UHFFFAOYSA-N
General description
The anion formed by metalation at the acetylenic carbon reacts with a variety of electrophiles.
Application
Tetrahydro-2-(2-propynyloxy)-2H-pyran was used in preparation of 1,3-dienyl acetal.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Aldrichimica Acta, 19, 9-9 (1986)
A facile synthetic method of a-quaternary-?, ?-unsaturated aldehydes via the stereoselective 1, 4-elimination and a-regioselective Ferrier reaction
Tayama E and Hashimoto R
Tetrahedron Letters, 48(45), 7950-7952 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service