291390
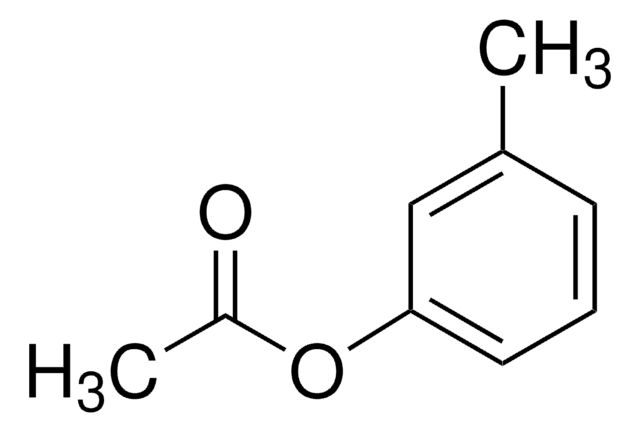
p-Tolyl acetate
99%
Synonym(s):
p-Cresyl acetate
About This Item
Recommended Products
Assay
99%
refractive index
n20/D 1.501 (lit.)
bp
210-211 °C (lit.)
density
1.047 g/mL at 25 °C (lit.)
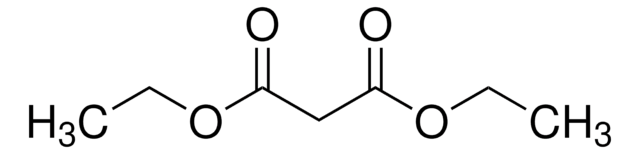
SMILES string
CC(=O)Oc1ccc(C)cc1
InChI
1S/C9H10O2/c1-7-3-5-9(6-4-7)11-8(2)10/h3-6H,1-2H3
InChI key
CDJJKTLOZJAGIZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
194.0 °F - closed cup
Flash Point(C)
90 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The Fries rearrangement reaction is an organic name reaction which involves the conversion of phenolic esters into hydroxyaryl ketones on heating in the presence of a catalyst. Suitable catalysts for this reaction are Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4, or SnCl4. The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service