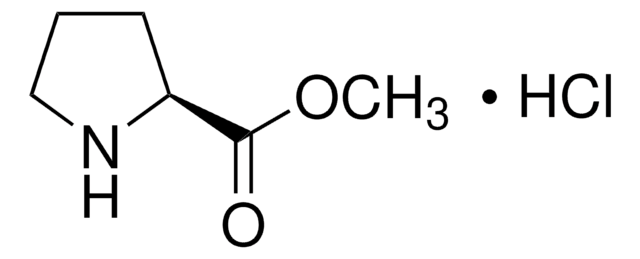
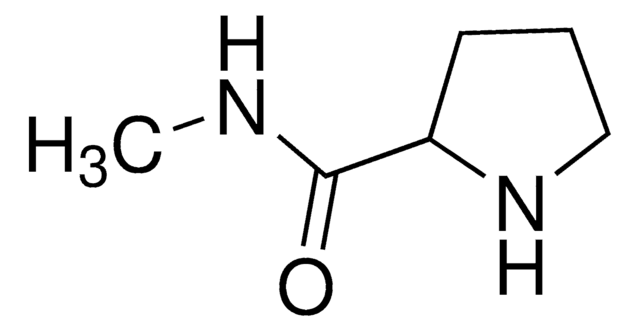
287059
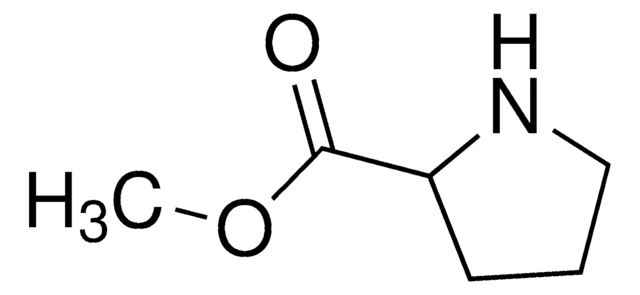
L-Prolinamide
98%, for peptide synthesis
Synonym(s):
(2S)-2-Carbamoylpyrrolidine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C5H10N2O
CAS Number:
Molecular Weight:
114.15
Beilstein:
80807
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
L-Prolinamide, 98%
Quality Level
Assay
98%
optical activity
[α]20/D −106°, c = 1 in ethanol
reaction suitability
reaction type: solution phase peptide synthesis
mp
95-97 °C (lit.)
application(s)
peptide synthesis
SMILES string
NC(=O)[C@@H]1CCCN1
InChI
1S/C5H10N2O/c6-5(8)4-2-1-3-7-4/h4,7H,1-3H2,(H2,6,8)/t4-/m0/s1
InChI key
VLJNHYLEOZPXFW-BYPYZUCNSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hidenobu Komeda et al.
European journal of biochemistry, 271(8), 1465-1475 (2004-04-07)
An amidase acting on (R,S)-piperazine-2-tert-butylcarboxamide was purified from Pseudomonas azotoformans IAM 1603 and characterized. The enzyme acted S-stereoselectively on (R,S)-piperazine-2-tert-butylcarboxamide to yield (S)-piperazine-2-carboxylic acid. N-terminal and internal amino acid sequences of the enzyme were determined. The gene encoding the S-stereoselective
Kazuhiko Mitsui et al.
Chemical communications (Cambridge, England), (22)(22), 3261-3263 (2009-07-10)
Dendritic effects on both the enantioselectivity and diastereoselectivity of the direct aldol reaction were observed for pyridine-2,6-dicarboxamide dendrons terminated with L-prolinamides.
Yasuhiro Goto et al.
Journal of medicinal chemistry, 49(3), 847-849 (2006-02-03)
A focused library approach identifying novel leads to develop a potent ORL1 antagonist is described. Beginning from a compound identified by random screening, an exploratory library that exhibited a diverse display of pharmacophores was designed. After evaluating ORL1 antagonistic activity
Sampak Samanta et al.
Organic letters, 7(23), 5321-5323 (2005-11-05)
[reaction: see text] The catalytic activity of the prolinamide-type catalysts may be improved by introducing additional prolinamide moiety into the catalyst, while the enantioselectivity can still be maintained or further improved. A C2-symmetric bisprolinamide with two prolinamide moieties has been
Highly enantioselective strecker reaction of ketoimines catalyzed by an organocatalyst from (S)-BINOL and L-prolinamide.
Zongrui Hou et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(15), 4484-4486 (2008-04-11)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service