281409
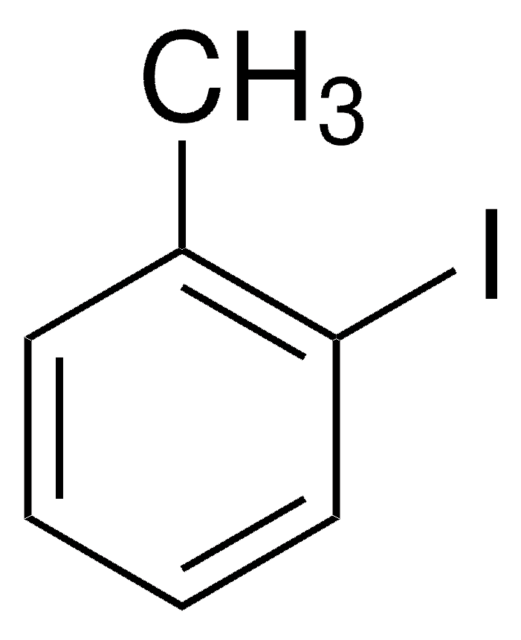
2-Iodophenol
98%
Synonym(s):
1-Iodo-2-hydroxybenzene, 2-Hydroxyiodobenzene, o-Iodophenol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
IC6H4OH
CAS Number:
Molecular Weight:
220.01
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
186-187 °C/160 mmHg (lit.)
mp
37-40 °C (lit.)
density
1.947 g/mL at 25 °C (lit.)
SMILES string
Oc1ccccc1I
InChI
1S/C6H5IO/c7-5-3-1-2-4-6(5)8/h1-4,8H
InChI key
KQDJTBPASNJQFQ-UHFFFAOYSA-N
General description
Rapid palladium-catalyzed carbonylative cyclization reactions of 2-iodophenol with various alkyne under microwave irradiation using Mo(CO)6 as the CO source has been investigated.
Application
2-Iodophenol was used in the synthesis of:
- aryl 2-benzofuranyl and aryl 2-indolyl carbinols of high enantiomeric purity
- 3,3-disubstituted-2,3-dihydrobenzofurans
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Microwave-accelerated, palladium-catalyzed carbonylative cyclization reactions of 2-iodophenol with alkynes Rapid and efficient synthesis of chromen-2-one derivatives.
Cao H and Xiao W-J.
Canadian Journal of Chemistry, 83(6-7), 826-831 (2005)
D M Otterness et al.
Molecular pharmacology, 36(6), 856-865 (1989-12-01)
Phenol sulfotransferase (PST) catalyzes the sulfate conjugation of phenolic drugs, neurotransmitters, and xenobiotic compounds. Human tissues contain at least two forms of PST, which differ in their substrate specificities, inhibitor sensitivities, physical properties, and regulation. One form of the enzyme
Chao Fang et al.
Water research, 145, 103-112 (2018-08-20)
Haloacetamides (HAMs), an emerging class of disinfection by-products, have received increasing attention due to their elevated cyto- and genotoxicity. However, only limited information is available regarding the iodinated analogues. This study investigated the formation and speciation of iodinated haloacetamides (I-HAMs)
J Philipp Wagner
Chemistry (Weinheim an der Bergstrasse, Germany), 26(53), 12119-12124 (2020-05-20)
Peroxy radical hydrogen-shifts are pivotal elementary reaction steps in the oxidation of small hydrocarbons in autoignition and the lower atmosphere. Although these reactions are typically associated with a substantial barrier, we demonstrate that the [1,5]H-shift in the peroxy species derived
Synthesis of aryl 2-benzofuranyl and aryl 2-indolyl carbinols of high enantiomeric purity via palladium-catalyzed heteroannulation of chiral arylpropargylic alcohols.
Botta M, et al.
Tetrahedron Asymmetry, 7(5), 1263-1266 (1996)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service