269697
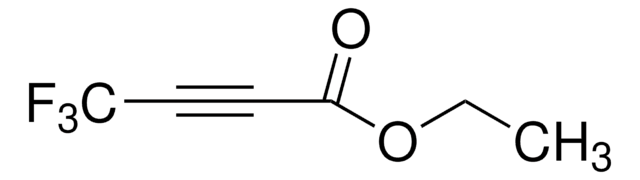
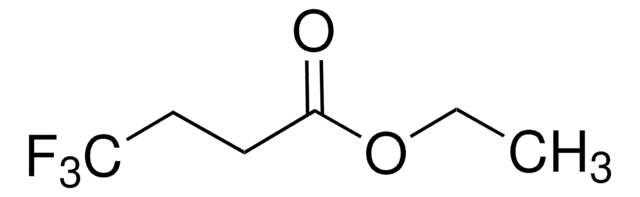
Ethyl 4,4,4-trifluorocrotonate
98%
Synonym(s):
Ethyl 4,4,4-trifluoro-2-butenoate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3CH=CHCO2C2H5
CAS Number:
Molecular Weight:
168.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.3601 (lit.)
bp
114-115 °C (lit.)
density
1.125 g/mL at 25 °C (lit.)
functional group
ester
SMILES string
CCOC(=O)\C=C\C(F)(F)F
InChI
1S/C6H7F3O2/c1-2-11-5(10)3-4-6(7,8)9/h3-4H,2H2,1H3/b4-3+
InChI key
ZKRJCMKLCDWROR-ONEGZZNKSA-N
General description
The Michael reaction between ethyl 4,4,4-trifluorocrotonate and a Ni(II) complex of the Schiff base of glycine with (S)-o-[N-(N-benzylprolyl)amino]benzophenone was studied.
Application
Ethyl 4,4,4-trifluorocrotonate was used in the synthesis of (2S,3S)-3-methyl- and (2S,3S)-3-trifluoromethylpyroglutamic acid. It undergoes diastereoselective Michael addition reaction with ethyl crotonate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
78.8 °F - closed cup
Flash Point(C)
26 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Stereochemically defined C-substituted glutamic acids and their derivatives. 1. An efficient asymmetric synthesis of (2S, 3S)-3-methyl-and-3-trifluoromethylpyroglutamic acids.
Soloshonok VA, et al.
Tetrahedron, 55(41), 12031-12044 (1999)
An efficient asymmetric synthesis of (2S,3S)-3-trifluoromethylpyroglutamic acid.
Tetrahedron Letters, 38(27), 4903-4904 (1997)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service