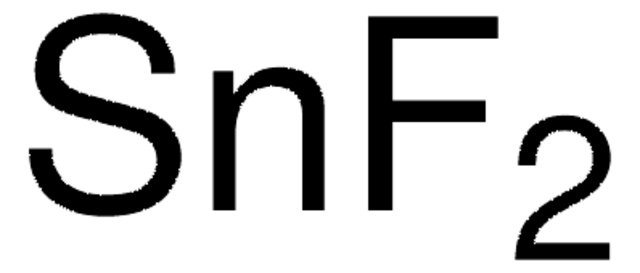265640
Tin
powder, <150 μm, 99.5% trace metals basis
Synonym(s):
Sn
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
Sn
CAS Number:
Molecular Weight:
118.71
EC Number:
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99.5% trace metals basis
form
powder
reaction suitability
core: tin
reagent type: catalyst
resistivity
11 μΩ-cm, 20°C
particle size
<150 μm
bp
2270 °C (lit.)
mp
231.9 °C (lit.)
density
7.310 g/mL at 25 °C (lit.)
SMILES string
[Sn]
InChI
1S/Sn
InChI key
ATJFFYVFTNAWJD-UHFFFAOYSA-N
Related Categories
General description
Tin is an efficient catalyst for the polycondensation of lactic acid to synthesize poly(lactic acid).
Application
With HCl, reduces a variety of functional groups; stereoselective allylation of carbonyl compounds; in situ generation of tin enolates for directed aldol reactions.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P Olmedo et al.
Environment international, 59, 63-72 (2013-06-25)
Although fish intake has potential health benefits, the presence of metal contamination in seafood has raised public health concerns. In this study, levels of mercury, cadmium, lead, tin and arsenic have been determined in fresh, canned and frozen fish and
Lun Li et al.
Journal of the American Chemical Society, 135(4), 1213-1216 (2013-01-15)
Single-layer single-crystalline SnSe nanosheet with four-atomic thickness of ~1.0 nm and lateral size of ~300 nm is presented here by using a one-pot synthetic method. It is found that 1,10-phenanthroline plays an important role in determining the morphology of the
Rutile-type (Ti,Sn)O₂ nanorods as efficient anode materials toward its lithium storage capabilities.
Yu-Chun Chen et al.
Nanoscale, 5(6), 2254-2258 (2013-02-13)
A series of rutile-type (Ti,Sn)O2 solid solutions with nanorod architecture were successfully synthesized in this study by varying their calcination temperatures of tin-modified titanium dioxide (Sn/TiO2) nanocomposites under a nitrogen atmosphere. During the delithiation process, the (Ti,Sn)O2 nanorods obtained at
Shiyou Chen et al.
Advanced materials (Deerfield Beach, Fla.), 25(11), 1522-1539 (2013-02-13)
The kesterite-structured semiconductors Cu2ZnSnS4 and Cu2ZnSnSe4 are drawing considerable attention recently as the active layers in earth-abundant low-cost thin-film solar cells. The additional number of elements in these quaternary compounds, relative to binary and ternary semiconductors, results in increased flexibility
D B Shpakovsky et al.
Dalton transactions (Cambridge, England : 2003), 41(48), 14568-14582 (2012-10-12)
Four new organotin(IV) complexes of bis-(2,6-di-tert-butylphenol)tin(IV) dichloride [(tert-Bu-)(2)(HO-Ph)](2)SnCl(2) (1) with the heterocyclic thioamides 2-mercapto-pyrimidine (PMTH), 2-mercapto-4-methyl-pyrimidine (MPMTH), 2-mercapto-pyridine (PYTH) and 2-mercapto-benzothiazole (MBZTH), of formulae {[(tert-Bu-)(2)(HO-Ph)](2)Sn(PMT)(2)} (2), {[(tert-Bu-)(2)(HO-Ph)](2)Sn(MPMT)(2)} (3), {[(tert-Bu-)(2)(HO-Ph)](2)SnCl(PYT)} (4) and {[(tert-Bu-)(2)(HO-Ph)](2)SnCl(MBZT)} (5), have been synthesized and characterized by elemental
Articles
Nanomaterials for Energy Storage in Lithium-ion Battery Applications
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service