All Photos(1)
About This Item
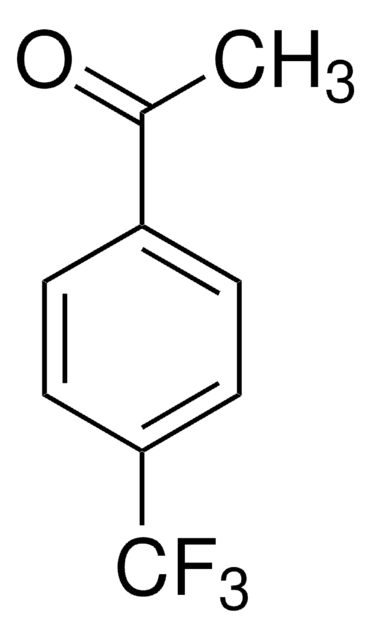
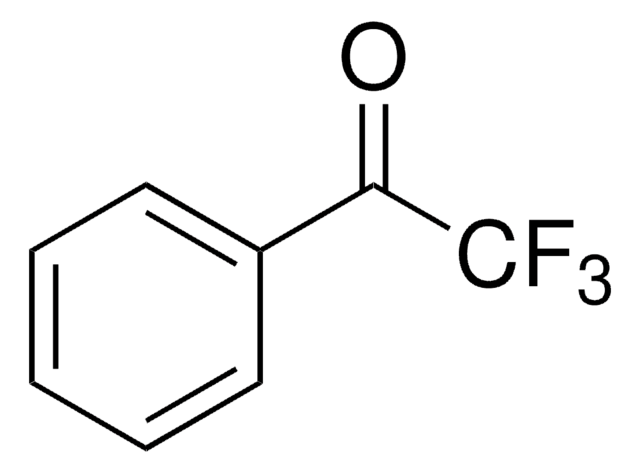
Linear Formula:
(CF3)2C6H3COCH3
CAS Number:
Molecular Weight:
256.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.4221 (lit.)
density
1.422 g/mL at 25 °C (lit.)
SMILES string
CC(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F
InChI
1S/C10H6F6O/c1-5(17)6-2-7(9(11,12)13)4-8(3-6)10(14,15)16/h2-4H,1H3
InChI key
MCYCSIKSZLARBD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
179.6 °F - closed cup
Flash Point(C)
82 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S Kneubühler et al.
Journal of medicinal chemistry, 38(19), 3874-3883 (1995-09-15)
A large series (66 compounds) of indeno[1,2-c]pyridazin-5-ones (IPs) were synthesized and tested on their monoamine oxidase-A (MAO-A) and MAO-B inhibitory activity. All of the tested compounds acted preferentially on MAO-B displaying weak (nonmeasurable IC50 values) to high (submicromolar IC50 values)
Rhodium-catalyzed heterogeneous enantioselective hydrogenation of 3, 5-di-(trifluoromethyl)-acetophenone.
Hess R, et al.
J. Mol. Catal. A: Chem., 212(1), 205-209 (2004)
Pu Wang et al.
Applied microbiology and biotechnology, 90(6), 1897-1904 (2011-05-27)
A novel bacterial strain HS0904 was isolated from a soil sample using 3,5-bis(trifluoromethyl) acetophenone as the sole carbon source. This bacterial isolate can asymmetrically reduce 3,5-bis(trifluoromethyl) acetophenone to (1R)-[3,5-bis(trifluoromethyl)phenyl] ethanol with high enantiometric excess (ee) value. Based on its morphological
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service