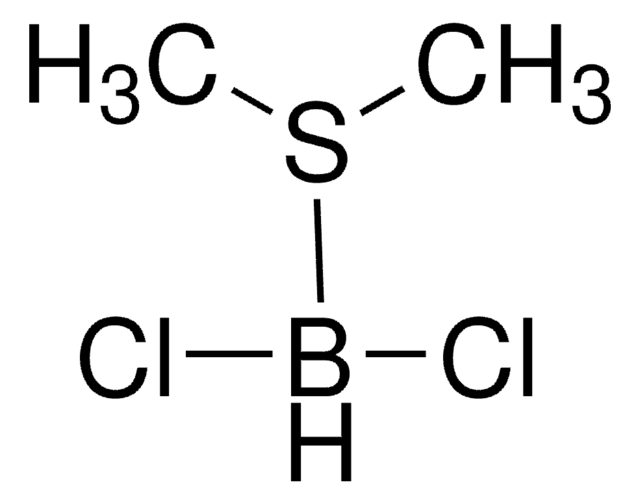
262064
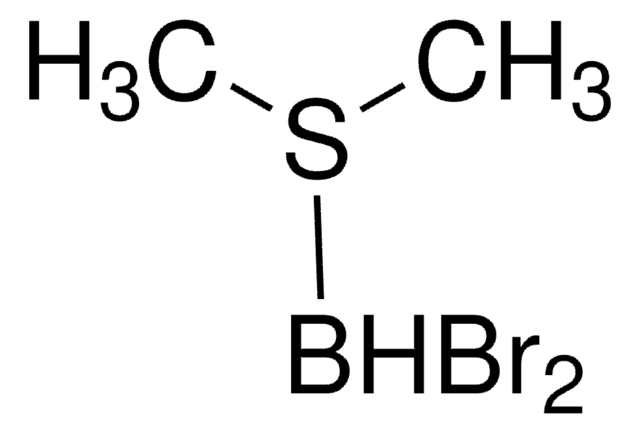
mono-Bromoborane methyl sulfide complex solution
1.0 M in methylene chloride
Synonym(s):
Bromo(dimethyl sulfide)borane, Bromo(dimethyl sulfide)dihydroboron
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2S·BH2Br
CAS Number:
Molecular Weight:
154.86
MDL number:
UNSPSC Code:
12352112
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
liquid
Quality Level
reaction suitability
reagent type: reductant
concentration
1.0 M in methylene chloride
density
1.347 g/mL at 25 °C
SMILES string
BBr.CSC
InChI
1S/C2H6S.BBrH2/c1-3-2;1-2/h1-2H3;1H2
InChI key
AQRNIOMHNXJPFD-UHFFFAOYSA-N
Related Categories
Application
Reactant for:
- Substitution reactions for synthesis of N-heterocyclic carbene boranes with boron-heteroatom bonds
- Hydroboration reactions
- Regio- and chemoselective brominative cleavage of terminal epoxides
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 1
Target Organs
Central nervous system
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
50.0 °F
Flash Point(C)
10 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xaba, N.; Jaganyi, D.
Polyhedron, 28, 1145-1145 (2009)
Roy, C. D.; Brown, H. C.
Australian Journal of Chemistry, 60, 139-139 (2007)
Andrey Solovyev et al.
Journal of the American Chemical Society, 132(42), 15072-15080 (2010-10-05)
Boryl halide, carboxylate and sulfonate complexes of 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene (dipp-Imd-BH(2)X, X = halide or sulfonate) have been prepared from the parent borane dipp-Imd-BH(3) by (1) substitution reactions with R-X (X = halide or sulfonate), (2) reactions with electrophiles (like I(2) or
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service