258563
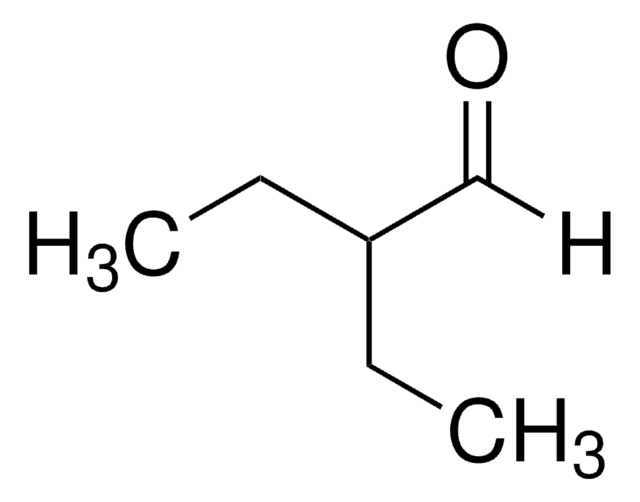
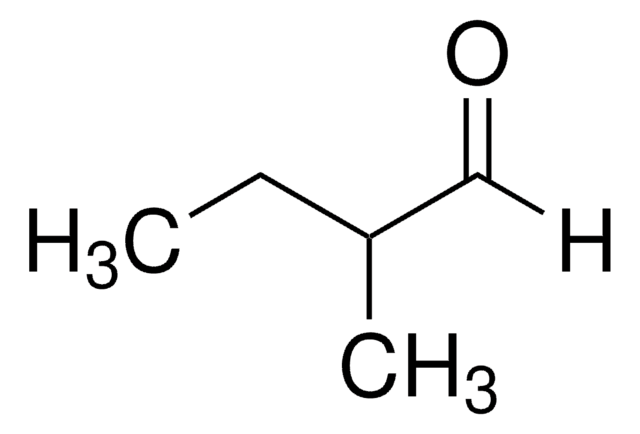
2-Methylpentanal
98%
Synonym(s):
2-Methylvaleraldehyde
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
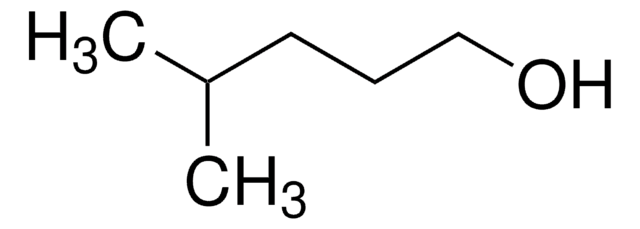
Linear Formula:
CH3CH2CH2CH(CH3)CHO
CAS Number:
Molecular Weight:
100.16
Beilstein:
1739423
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
3.45 (vs air)
Quality Level
Assay
98%
form
liquid
autoignition temp.
390 °F
refractive index
n20/D 1.401 (lit.)
bp
119-120 °C (lit.)
density
0.808 g/mL at 25 °C (lit.)
SMILES string
CCCC(C)C=O
InChI
1S/C6H12O/c1-3-4-6(2)5-7/h5-6H,3-4H2,1-2H3
InChI key
FTZILAQGHINQQR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Cross-aldol condensation reaction of 2-methylpentanal with propanal to form 2,4-dimethylhept-2-enal has been investigated. Oxidation of 2-methylpentanal with azidotrimetlzylsilane and chromic anhydride in CH2Cl2 has been investigated.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
60.8 °F - closed cup
Flash Point(C)
16 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Oxidation of aldehydes to acyl azides by chromic anhydride-azidotrimethylsilane.
Lee JG and Kwak KH.
Tetrahedron Letters, 33(22), 3165-3166 (1992)
Christian P Mehnert et al.
Chemical communications (Cambridge, England), (15), 1610-1611 (2002-08-13)
C9-aldehyde has been prepared via aldol condensation reactions in ionic liquid media; catalyst investigation showed enhanced product selectivity for the desired aldehyde in ionic liquid media than in conventional solvent systems.
Sajjad Ahmad et al.
Drug design, development and therapy, 15, 1299-1313 (2021-04-02)
Organocatalytic asymmetric Michael addition is a strong approach for C-C bond formation. The objective of the study is to design molecules by exploiting the efficiency of Michael Adducts. We proceeded with the synthesis of Michael adducts by tailoring the substitution
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service