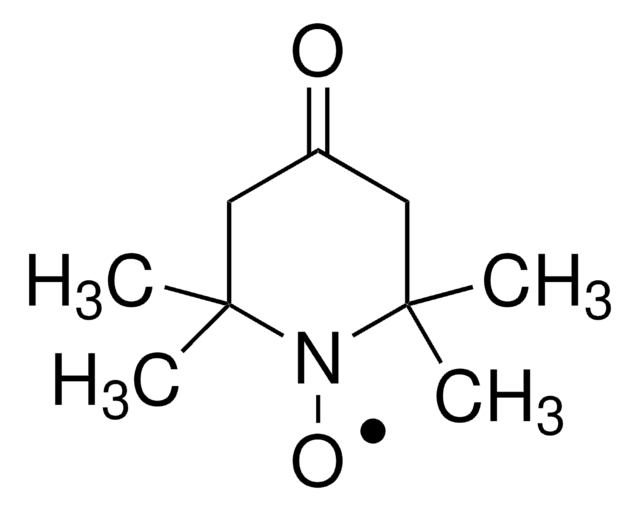
253421
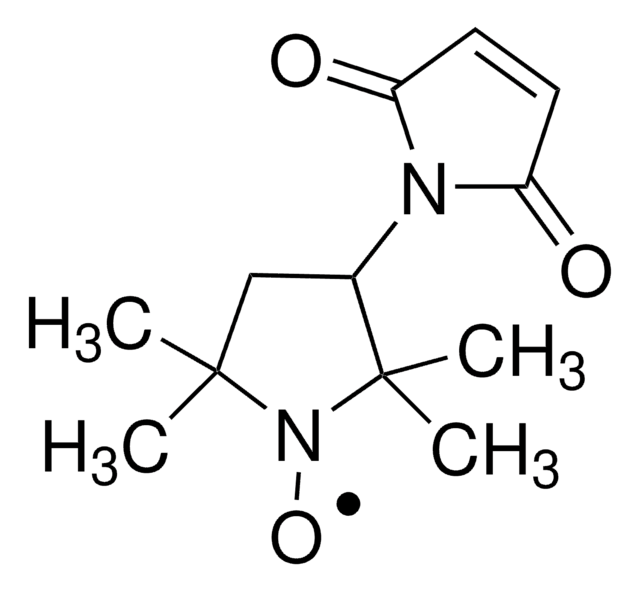
3-(2-Iodoacetamido)-PROXYL
free radical
Synonym(s):
3-(2-Iodoacetamido)-2,2,5,5-tetramethyl-1-pyrrolidinyloxy, free radical
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H18IN2O2
CAS Number:
Molecular Weight:
325.17
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
mp
162-164 °C (lit.)
storage temp.
2-8°C
SMILES string
CC1(C)CC(NC(=O)CI)C(C)(C)N1[O]
InChI
1S/C10H18IN2O2/c1-9(2)5-7(12-8(14)6-11)10(3,4)13(9)15/h7H,5-6H2,1-4H3,(H,12,14)
InChI key
OQIGMSGDHDTSFA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-(2-Iodoacetamido)-PROXYL is a free radical used as a spin probe in spin labeling techniques. This technique is helpful in investigating the dynamic aspects of molecular interactions of substances, proteins, lipids, and cell membranes.
Application
Sulfhydryl group specific spin label. Applications include study of ozone-treated human and bovine erythrocyte membrane proteins and the orientation of myosin subfragment on muscle fibers. DNA fragments containing phosphorothioate diesters can be labeled by spin labels.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
First spin-labeled cytisine derivatives
Polienko YF, et al.
Chemistry of Natural Compounds, 49(2), 311-315 (2013)
Claire Bagnéris et al.
Nature communications, 4, 2465-2465 (2013-09-21)
Voltage-gated sodium channels have essential roles in electrical signalling. Prokaryotic sodium channels are tetramers consisting of transmembrane (TM) voltage-sensing and pore domains, and a cytoplasmic carboxy-terminal domain. Previous crystal structures of bacterial sodium channels revealed the nature of their TM
Site directed nitroxide spin labeling of oligonucleotides for NMR and EPR studies
Shepherd NE, et al.
Tetrahedron, 71(5), 813-819 (2015)
Christoph Gmeiner et al.
Physical chemistry chemical physics : PCCP, 19(41), 28360-28380 (2017-10-17)
A combined method, employing NMR and EPR spectroscopies, has demonstrated its strength in solving structures of protein/RNA and other types of biomolecular complexes. This method works particularly well when the large biomolecular complex consists of a limited number of rigid
Na Li et al.
Scientific reports, 10(1), 393-393 (2020-01-17)
Fowlpox virus resolvase (Fpr) is an endonuclease that cleaves a broad range of branched DNA structures, including the Holliday junction (HJ), with little sequence-specificity. To better understand the mechanisms underlying its relaxed substrate specificity, we determined the crystal structures of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service